|
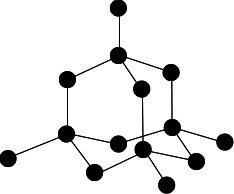
Chemguide: Core Chemistry 14 - 16 Giant covalent structures This page looks at the way some atoms arrange themselves into giant covalent structures, and the effect this has on their simple properties. It includes silicon dioxide (silica), and carbon as diamond, graphite and graphene. I am assuming that you already know about covalent bonding. What is a giant covalent structure? A giant covalent structure is one in which the atoms are joined up by covalent bonds over huge (but variable) numbers of atoms. It is not a molecule, because the number of atoms joined up in a diamond, say, is completely variable - depending on the size of the crystal. You may find that these structures are sometimes described as "giant molecular structures". I think this is misleading. A molecule contains a fixed number of atoms (even if that number is very large). In a giant covalent structure like diamond the number of carbon atoms is entirely variable. The giant covalent structure of diamond Carbon has an electronic arrangement of 2,4. In diamond, each carbon atom shares electrons with four other carbon atoms - forming four single bonds.
In the diagram some carbon atoms only seem to be forming two bonds (or even one bond), but that's because we are only showing a small part of the whole structure. Every carbon atom, until you get a crystal edge, will be joined to four others. The diagram above is the one you will see most often at this level, but it is slightly misleading. Apart from the fact that some of the atoms don't seem to be forming enough bonds, it is all too neat and tidy! There is a useful bit of YouTube video which shows a much bigger section of the structure. Nobody is suggesting that you should learn this or try to draw it, but it is interesting to see a more realistic part of the structure. | |
|
Note: You will, however, almost certainly need to be able to draw the diagram above. You will find instructions on how to draw this structure by following this link. | |
|
The physical properties of diamond Diamond
The giant covalent structure of graphite The photo shows lumps of graphite and the marks they will make on paper.
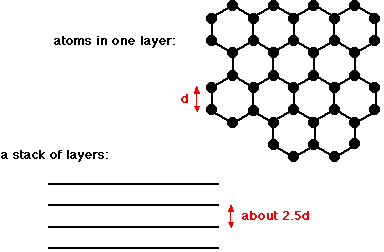
Graphite is another allotrope of carbon. Allotropes are different forms of the same element in the same physical state. So carbon and diamond are both allotropes of carbon, because they are both chemically carbon, both solids, but obviously different. Graphite has a layer structure which is quite difficult to draw convincingly in three dimensions. The diagram below shows the arrangement of the atoms in each layer, and the way the layers are spaced.
Notice that you can't really draw the side view of the layers to the same scale as the atoms in the layer without one or other part of the diagram being either very spread out or very squashed. In that case, it is important to give some idea of the distances involved. The distance between the layers is about 2.5 times the distance between the atoms within each layer. The layers, of course, extend over huge numbers of atoms - not just the few shown above. And there are huge numbers of layers in each stack. You might argue that carbon has to form 4 bonds because of its 4 unpaired electrons, whereas in this diagram it only seems to be forming 3 bonds to the neighbouring carbons. This diagram is something of a simplification, and shows the arrangement of atoms rather than the bonding. The bonding in graphite Each carbon atom uses three of its electrons to form simple covalent bonds to its three close neighbours. That leaves a fourth electron in the bonding level. These "spare" electrons in each carbon atom become delocalised over the whole of the sheet of atoms in one layer. They are no longer associated directly with any particular atom or pair of atoms, but are free to wander throughout the whole sheet. The important thing is that the delocalised electrons are free to move anywhere within the sheet - each electron is no longer fixed to a particular carbon atom. There is, however, no direct contact between the delocalised electrons in one sheet and those in the neighbouring sheets. The atoms within a sheet are held together by strong covalent bonds - stronger, in fact, than in diamond because of the additional bonding caused by the delocalised electrons. The forces of attraction between the layers are much weaker. The physical properties of graphite Graphite
| |
|
Note: The logic of this is that a piece of graphite ought only to conduct electricity in 2-dimensions because electrons can only move around in the sheets - and not from one sheet to its neighbours. In practice, a real piece of graphite isn't a perfect crystal, but a host of small crystals stuck together at all sorts of angles. Electrons will be able to find a route through the large piece of graphite in all directions by moving from one small crystal to the next. | |
|
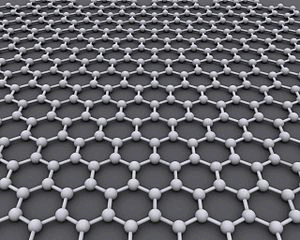
The giant covalent structure of graphene I am simply flagging up the existence of graphene, but without going into detail. Graphene is just a single sheet of the graphite structure.
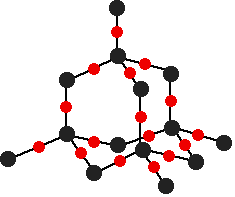
The photo comes from this Wikipedia page. If you want to follow this up, you will probably find that page has far more than you want to know about graphene, and is far too complicated. A Google search should find something more accessible. The giant covalent structure of silicon dioxide, SiO2 This is probably less likely to come up in exams at this level then diamond and graphite, and I include it only to show that there are giant covalent substances which contain something other than carbon. Silicon dioxide (also known as silica) is an important component of various rocks. There are various different crystal forms of silicon dioxide. The easiest one to remember and draw is based on the diamond structure. Crystalline silicon has the same structure as diamond. To turn it into silicon dioxide, all you need to do is to modify the silicon structure by including some oxygen atoms.
Notice that each silicon atom is bridged to its neighbours by an oxygen atom. Don't forget that this is just a tiny part of a giant structure extending on all 3 dimensions. | |
|
Note: If you want to be fussy, the Si-O-Si bond angles are wrong in this diagram. In reality the "bridge" from one silicon atom to its neighbour isn't in a straight line, but via a "V" shape (similar to the shape around the oxygen atom in a water molecule). It's extremely difficult to draw that convincingly and tidily in a diagram involving this number of atoms. The simplification is perfectly acceptable. | |
|
The physical properties of silicon dioxide Silicon dioxide
© Jim Clark 2019 |
|