|
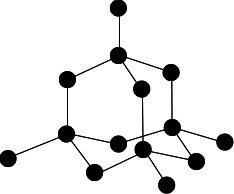
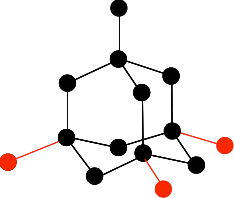
Chemguide: Core Chemistry 14 - 16 How to draw a simple diamond structure This page is an add-on to a page about giant covalent structures. It simply describes how to draw the structure of a diamond lattice. This is the diagram we are aiming at:
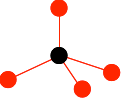
Draw a carbon atom, and then arrange 4 others around it to look like this:
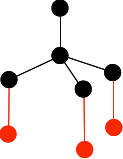
It is important that you don't draw the ones at the bottom so that they are exactly lined up with any other atom either vertically or horizontally. The arrangement around the central carbon atom is described as tetrahedral. A tetrahedron is a pyramid with a triangular base. You can picture the black carbon as being in the centre of the tetrahedron, and the red ones on the corners. Now draw three more carbon atoms directly underneath the bottom ones in the last diagram.
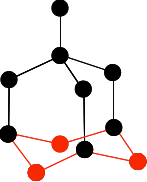
The next bit is the tricky one to get right. You have to bridge the the three bottom carbons in the previous diagram with three more carbons creating rather bent hexagons.
Make sure you don't get any of the new ones tangled up (or overlapping) with any of the ones you have drawn before. Finally, add the last three carbon atoms.
You should aim to be able to draw an acceptable clear sketch of this in no more than about 30 seconds. © Jim Clark 2019 |