|
Chemguide: Core Chemistry 14 - 16 Catalysts This page looks at some reactions speeded up by catalysts, and explains how the catalysts achieve that. I am assuming that you will already have read the page about the effect of temperature on rates of reaction which introduces the concept of activation energy. What is a catalyst?
When the reaction has finished, you would have exactly the same mass of catalyst as you had at the beginning. How does a catalyst work? Reactions happen when molecules collide with at least enough energy to equal activation energy. In less energetic collisions, the particles just bounce off each other.
What does that mean? Chemical reactions are often far more complicated in terms of what is happening to the individual atoms than you might suppose. When a catalyst gets involved in a reaction, it gives the reactants another way of getting to the same products, but by a much easier method involving less activation energy. There is a very nice example of this involving a reaction which nobody would expect you to know at this level. I'm talking about it because it gives a very clear explanation of how a particular catalyst works. The reaction between persulfate ions and iodide ions This reaction happens in solution. I have left the state symbols out because they aren't really relevant.
This is a slow reaction, and it is pretty obvious why. For the reaction to happen, at some point two negative ions will have to hit each other and react - but they are going to repel each other. The reaction is catalysed by adding a solution containing iron(II) ions, Fe2+. The reaction can now happen in two stages both of which involve reactions between a negative and a positive ion.
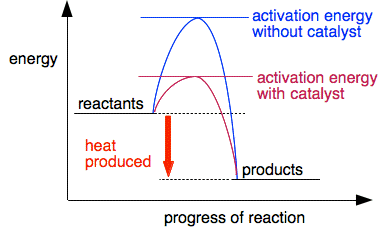
Notice that we have the same overall products (sulfate ions and iodine). And, importantly, the iron(II) ions which were changed to iron(III) ions in the first stage have been changed back into iron(II) ions by the end. So the catalyst has speeded up the reaction and is chemically unchanged at the end of the reaction. Important! I stress that you don't need to remember this reaction. It is just an example of what it means for a reaction to go by an alternative route. Catalysts and reaction profiles We've seen that a catalyst provides an alternative route of lower activation energy. You can show this on an energy profile for the reaction.
Because the activation energy for the catalysed reaction is lower, a lot more collisions will have enough energy to equal or exceed the activation energy, and so the reaction will be much faster. | |
|
Note: You might think that because the activation energy has dropped by a bit more than half in the diagram, the number of particles reaching activation energy will have a bit more than doubled. It isn't as simple as that - you get a very large increase in the number of sufficiently energetic particles. Understanding this is a matter for chemistry at a higher level, but if you are interested, you could read about the Maxwell-Boltzmann distribution which you can find introduced here and related to catalysis here. You don't need to know any of this at the present level. | |
|
Some experimental examples Start by watching this bit of video. Notes I don't want to say much about the first experiment using manganese(IV) oxide (manganese dioxide) to catalyse the decomposition of hydrogen peroxide. We are going to look at that again further down the page. The second experiment is an easy visual way of comparing possible catalysts. The third experiment with a cobalt(II) compound shows that a catalyst is changed during the course of a reaction, but ends up the same as it started when the reaction is complete. This is a wonderful reaction that everyone should see. If you are a teacher who hasn't come across it before there is a detailed description and explanation on this site. The fourth reaction involving copper(II) oxide catalysing the decomposition of potassium chlorate isn't giving you all the necessary information to do the experiment. When you filter the solution to leave the copper(II) oxide on the filter paper, you can't just allow it to dry. The paper is soaked in potassium chloride solution - the other product from the reaction.
You would have to wash the filter paper and contents several times with pure water to remove that before allowing it to dry. The catalytic decomposition of hydrogen peroxide using MnO2
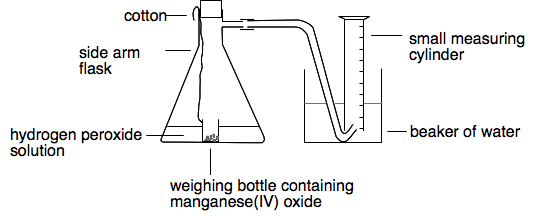
Notice that the manganese(IV) oxide isn't written into the overall equation for this reaction. If you want to include it, you can write it over the top of the arrow. That is a common way of showing something is a catalyst. You saw this reaction twice on the video, but I want to look in detail at a more accurate way of doing it. Notes on the apparatus
This is a useful and accurate way of measuring the initial rate of a reaction involving the formation of a gas from a mixture of a liquid and a solid. The problem with an experiment like this is either losing a small amount of gas before you can get the bung into the flask, or forcing some extra air into the measuring cylinder when you push the bung in.
This gets around both of these problems. Doing the reaction with hydrogen peroxide solution and MnO2 You measure a known volume of hydrogen peroxide solution into the flask. You measure the amount of manganese(IV) oxide you want to use into a small weighing bottle on the end of a bit of cotton, and carefully lower it into the flask with the liquid in, taking care to keep it upright. Then you put the bung in before you place the measuring cylinder over the delivery tube. When you are ready to start, you shake the flask around so that the bottle falls over and the solid gets mixed with the liquid. Then record the time taken for a small volume of oxygen to be formed - say, 5 cm3. That lets you calculate an initial rate of reaction in terms of cm3 of oxygen per second. Now you can change the conditions and repeat the experiment. You could:
In each case you must only change one variable at a time. For each change of variable, you would need to do a series of experiments - for example, at various temperatures, or various concentrations of the hydrogen peroxide solution. Processing the results For this part, I am assuming that you have read the page about the effect of concentration on rates of reaction. In the present case, finding the initial rate is easy - you are getting it directly from the experiment. For example if it takes 20 seconds to collect 5 cm3 of gas, the initial rate is 5/20 = 0.25 cm3 per second. So let's look at the variations I suggested above. Varying the mass of catalyst used. Since the reaction only occurs on the surface of the solid, you are essentially varying the mass to vary the surface area. Assuming you have a powder and have mixed it thoroughly with the solution, if you plot a graph of rate (y-axis) against mass (x-axis), it would seem reasonable to expect a straight line. Surface area will be proportional to the mass you are using - the greater the mass, the greater the surface area, and the faster the reaction. Varying the concentration of the hydrogen peroxide solution. You will find that the rate of reaction is proportional to the concentration of the hydrogen peroxide. If you plotted rate (y-axis) against concentration (x-axis), you would expect a straight line passing through the origin. (If you have zero concentration, then the rate will be zero.) Varying the temperature of the reaction. If you plot rate (y-axis) against temperature (x-axis), this time you will get a curve getting increasingly steeper at higher temperatures. You should find that the rate about doubles for every 10°C rise in temperature. Finally . . . You should treat this last bit as a useful summary of the main effects of changing conditions on the rate of a reaction. It brings together all the factors (apart from pressure) we have looked at throughout this topic.
© Jim Clark 2020 |
|