|
Chemguide: Core Chemistry 14 - 16 Diffusion This page looks at examples of diffusion, mainly in gases but also in liquids. It shows how these can easily be explained in terms of particles and their movement. Diffusion in gases A simple example involving hydrogen I am not going to start with a definition of diffusion. Once we have looked at a simple example, it is so obvious that it hardly needs defining. 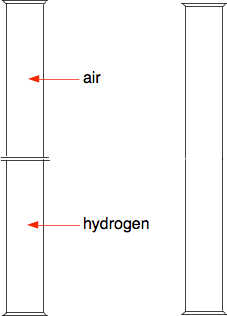
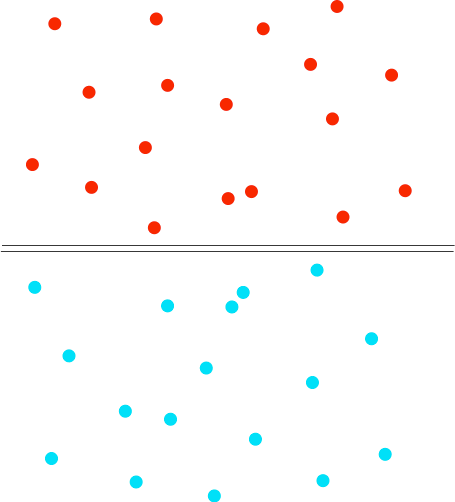
Suppose you have a gas jar full of hydrogen, and one with just air in it. To start with there are lids on both gas jars so that nothing can mix. Then we remove the lids. Everything is colourless and so we won't see anything happening, but it is easy to find out where the hydrogen ends up - we can test it with a lighted splint or a lighted taper. Most people know that hydrogen is a light gas, and so students often predict that it will be in the top gas jar. And if you test the top gas jar you get a satisfying bang. But then if you test the bottom gas jar as well, you get an equally satisfying bang. Students now suggest that perhaps you didn't leave it long enough for all the hydrogen to rise. So you repeat the experiment reversing the hydrogen and air - this time with the hydrogen at the top and the air at the bottom. When you test the two gas jars, you again get an equally satisfying bang from both of the jars. So what is happening? The diagram shows the particles in two gases separated from each other. The particles in each gas are moving around at random. They will collide with each other, changing direction and speeds - some will speed up, others will slow down.
What happens when you remove the barrier? The particles just keep on doing what they were doing before - moving around at random, colliding, and changing speeds and direction when they collide. The collisions now, of course, include collisions between different coloured particles. Given a bit of time, you will end up with an even mixture throughout the two gas jars. This spreading out of particles in a gas (or a liquid as well) because of the random motion of the particles is known as diffusion. | |
|
Note: Diffusion is often defined as the movement of a substance from an area of high concentration to an area of low concentration. I am very reluctant to use this, because it doesn't stress the randomness of the process. The statement is true, but suggests that there is some organisation in the movement - "We are a bit squashed together here - let's move over there where there is a bit more space!" All that is actually happening is that a substance spreads out because of the random motion of its particles. If you might have to explain what you mean by diffusion in an exam, check to find out what your examiners want you to say by looking at past papers and mark schemes. And beware! I wouldn't be surprised to find it differs depending on whether you are doing chemistry or physics or biology. | |
|
So doesn't the fact that hydrogen is a lighter gas matter? On the gas jar scale, not really. Gravity will have less effect on the hydrogen particles because they are lighter than the air particles, but hydrogen particles are also much faster moving - the lighter the particles the faster their average speed. If a hydrogen particle finds itself at the top of the gas jars, it isn't going to take very long before it has randomly bounced its way back to the bottom again. Repeating this with bromine and air You can see this happening if you use bromine vapour and air rather than hydrogen and air. Bromine vapour is brown, and bromine particles are heavier than air particles. If you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. It takes a while because bromine particles are relatively slow moving, but eventually you will have an even brown colour throughout the gas jars. If you start with the bromine in the top jar, then gravity helps, and the mixing is more rapid - but the final even colour is still the same. Showing that different particles move at different speeds Ammonia gas and hydrogen chloride gas are both colourless gases, but when they mix you get a white smoke. They react to form solid white ammonium chloride. Concentrated ammonia solution gives off colourless fumes of ammonia. Concentrated hydrochloric acid is a solution of hydrogen chloride in water, and gives off colourless fumes of hydrogen chloride. If you pour some of each into two side-by-side beakers, you can see the white smoke forming.
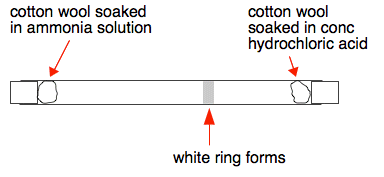
This next experiment does this reaction in a long glass tube. A piece of cotton wool soaked in ammonia solution is put in one end of the tube at the same time that another piece soaked in concentrated hydrochloric acid is placed in the other. After half a minute or so (depending on how long the tube is) a white ring forms closer to the hydrochloric acid end.
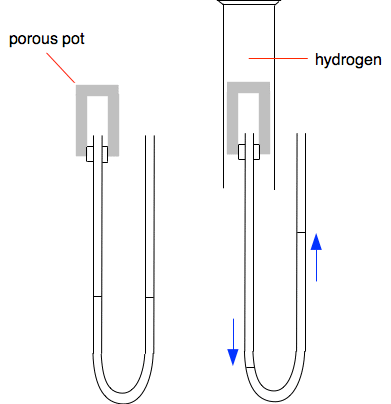
Why does the ring form closer to the hydrochloric acid end? Because the ammonia particles cover a greater distance than the hydrogen chloride particles in the time. Ammonia particles move faster than the hydrogen chloride particles. In fact it is possible to work out the speeds of these particles (although not at this level). At room temperature ammonia particles travel at around 650 metres per second on average; hydrogen chloride at about 450 metres per second. This is due to ammonia particles being lighter than hydrogen chloride particles. The lighter the particle, the faster its average speed. So why does it take so long for the ring to form? If it took 30 seconds, say, for the ring to form, the ammonia particles will have travelled almost 20000 metres - 20 kilometres - in that time! The reason, of course, is that the particles aren't moving in straight lines - they are constantly colliding with air particles in the tube. You will find a useful bit of video towards the bottom of the page which includes an experiment showing what happens if bromine vapour diffuses into a vacuum. Showing that particles in hydrogen move faster than those in air This demonstration isn't anything like as common as the previous one, but is interesting both to do and explain. The left-hand diagram shows a porous pot connected to a U-tube containing water, or sometimes water with some colour added to make it more visible. A porous pot is made of unglazed ceramic and, although it looks solid, at the microscopic level is full of small holes. It allows gases to diffuse through it, although more slowly than they would otherwise.
Initially the levels in the U-tube are identical on both sides showing that the pressure in the porous pot is the same as in the air on the other arm of the U-tube. That is what you would expect. If you put a gas jar of air over the porous pot, absolutely nothing happens - again that is just what you would expect. But if you put a gas jar of hydrogen over the porous pot, there is a sudden large movement in the levels in the U-tube as shown in the second diagram. Why? Hydrogen particles are smaller, lighter and much faster moving than particles in the air. There are hydrogen particles diffusing into the porous pot, and air particles diffusing out - but the hydrogen particles move faster. That means that there will quickly be a build-up of particles inside the pot. Pressure is caused by particles hitting the walls of the container or, in addition in this case, the surface of the water in the U-tube. In the other arm of the U-tube, there are the same number of air particles as before hitting the surface on that side. The extra hydrogen particles push the water level down on the left-hand side of the tube. What will happen when you remove the gas jar of hydrogen? Think it out! The porous pot now has lots of hydrogen particles inside it. These will diffuse out faster than new air particles can diffuse in. That causes the pressure to drop, and the U-tube levels swing back the other way, over-shooting their original levels to a small extent. Eventually, all the hydrogen will have diffused out to be replaced by air particles, and the pressure will drop back to what it was in the beginning. So the water levels will slowly return to their original positions. Diffusion in liquids Diffusion in liquids is much slower than in gases, because there isn't much space between the particles in a liquid for fast movement to occur. The particles diffusing are often much bigger and heavier as well which makes them slower moving. If you carefully drop some ink or food colouring (both containing large particles) into a glass of perfectly still water, it can take quite a long time for the colour to spread evenly throughout the whole liquid. A useful (although flawed) piece of video This demonstrates some of the things we have talked about. You have to bear in mind that it was first made decades ago, and it has a terrible bit of animation supposedly showing the particles in two gases mixing. Ignore that!
© Jim Clark 2018 |
|