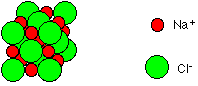|
Chemguide: Core Chemistry 14 - 16 Elements, compounds and mixtures This page explains the difference between an element and a compound, and how both of these differ from mixtures. It also takes a quick look at the Periodic Table - a really important tool in chemistry. Elements What is an element? I am going to include some chemical formulae in what comes next. Don't worry about it if you haven't done any work on symbols yet. They are just there to illustrate the complexity of various substances. Cu is the symbol for copper; the others are fairly obvious. If you heat some blue copper(II) nitrate crystals, Cu(NO3)2,3H2O, you get a chemical change and it turns into black copper(II) oxide, CuO, giving off nitrogen dioxide, oxygen and steam in the process. Copper(II) oxide is obviously a simpler substance than copper(II) nitrate. If you pass hydrogen over hot copper(II) oxide, the hydrogen removes the oxygen, and leaves pinkish-brown copper, Cu. Again copper is a simpler substance than copper(II) oxide. But there is nothing you can do chemically to turn copper into anything still simpler. Copper is an element. Elements are substances which can't be split into anything simpler by chemical means. The Periodic Table The Periodic Table lists all the known elements in a particular order which we will talk about in a minute. I have simplified it by leaving out elements that you aren't likely to come across at this level.
| |
|
Note: You really need a full-sized paper copy of a Periodic Table which you can read easily. You will find a simple Periodic Table which you can download from this site. This doesn't have all the most recently discovered elements, but it has more than you will ever need at this level. | |
|
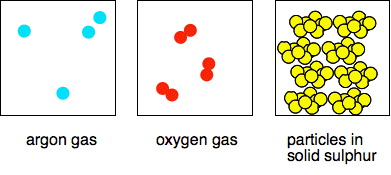
So . . . if something appears in a full version of the Periodic Table it is an element; otherwise it isn't. Elements and atoms If you had some copper and tried to chop it up into smaller and smaller pieces, eventually you would end up with the smallest possible bit of copper. At that point you would have an individual copper atom. Atoms themselves are made up of even smaller particles (protons, neutrons and electrons), but if you split the copper atom into those, you wouldn't have copper any longer. And in any case, you can't split atoms by chemical means. The protons and neutrons are found packed together in the nucleus at the centre of the atom and make up most of the mass of the atom. The electrons surround the nucleus at relatively large distances. Atomic number (proton number) The number of protons in an atom is known as the atomic number or proton number. If you look at the Periodic Table above, the little numbers in each box are the atomic numbers of the elements. The Periodic Table is arranged in order of increasing atomic numbers. The really important thing is that the number of protons defines what the atom is. For example, if an atom has 8 protons it is an atom of oxygen, and every oxygen atom contains 8 protons. A fuller definition of an element Elements are substances which can't be split into anything simpler by chemical means. (We have already said that.) A substance is an element if all the atoms present have the same atomic number. Some examples of elements In models or diagrams If all the atoms in a model or diagram are the same size and colour, then we assume that they are all the same - they have the same atomic number. If everything present has the same atomic number, then it is an element. It doesn't matter how the atoms are arranged, whether singly or in pairs or in large indefinite groups - if they are all the same size and colour, then the diagram is showing an element. Examples where the atoms are in small well-defined groups
| |
|
Note: For ease of drawing, all the particles above are not to scale. Individual sulfur atoms, for example, are bigger than oxygen atoms. The packing in the solid sulfur isn't accurate either and is just to suggest that the particles are closely packed together. The packing of the S8 units is actually very complicated, and difficult to understand from the only diagram I could find. | |
|
Another important word: molecule. In the diagrams above, the two oxygen atoms joined together form a molecule of oxygen. The eight sulfur atoms form a sulfur molecule. A molecule is an electrically neutral particle made up of a fixed number of atoms chemically bonded together. If the atoms in a molecule all have the same atomic number, then the substance is an element. If they have different atomic numbers, then it is a compound (see below). Examples of large indefinite groups - giant structures 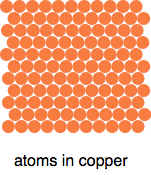
Metals like copper don't have any fixed number of atoms in their structures. You can break bits off, and you just have smaller bits of copper. The diagram shows an idealised bit of copper. In reality, there will be distortions in the structure where everything doesn't line up so tidily. But the important point is that if all the atoms are the same (as they are here), it is an element. 
Diamond is another giant structure, this time of carbon atoms, and the diagram shows a very small part of it. The diagram shows the bonds joining the carbon atoms - in reality the carbon atoms are all touching their near neighbours. It is virtually impossible to draw a useful diagram of the diamond structure without separating the atoms out in this way. Each carbon atom is attached to four others, continuing over vast numbers of atoms. In the diagram, you mustn't assume that some carbons only have two bonds - they have four, but for simplicity the other two aren't shown. Again, there is only one type of atom present, and so it is an element. Examples of elements in chemical formulae If a formula only contains one sort of symbol, then it is an element. For example, the formulae Ar, H2, O2, P4, S8, C and Cu all contain single types of symbol. These are all elements. If it contains more than one sort of symbol, then it is not an element. For example, the formulae NaCl, H2O, NH3, C3H8 all contain more than one sort of atom, so aren't elements. These are actually all compounds, and we are just about to have a look at these. Compounds What is a compound? A compound consists of two or more different elements chemically combined together. Some compounds are made up of molecules, where some fixed number of atoms are bonded to each other. Others consist of giant structures containing huge, but variable, numbers. Examples of compounds containing molecules Water, H2O, is a simple example of a compound. It has two hydrogen and one oxygen atom combined together, and that is always the case. There is another compound of hydrogen and oxygen called hydrogen peroxide, H2O2, but that is chemically quite different from water. Hydrogen peroxide again always has this ratio of hydrogen and oxygen atoms.
Another two simple compounds, also involving hydrogen atoms, are ammonia and propane.
There are, in fact, a vast number of other compounds of carbon and hydrogen. An example of a compound containing a giant structure Common salt, sodium chloride, NaCl, is a good example of a compound which consists of a giant structure. In fact, sodium chloride doesn't contain simple sodium and chlorine atoms, but instead contains sodium and chloride ions. Ions are atoms or groups of atoms which have gained an electric charge. In this case, sodium has formed a positive ion, Na+, while chlorine has formed a negative ion, Cl-.
| |
|
Note: When chlorine is a simple atom or a molecule, Cl2, it is just called chlorine. When it is in a compound such as sodium chloride or hydrogen chloride, the name changes to chloride. | |
|
The sodium chloride is held together by the attractions between the positive and negative ions. The diagram shows a small part of the giant structure - which just keeps going in all three directions until it gets to an edge where you have broken a bit off.
However big a salt crystal is, the ratio of sodium to chloride ions is always 1:1. Mixtures Copper(II) nitrate crystals compared with a mixture of its elements nitrate.jpg)
At the top of this page, I talked about blue copper(II) nitrate crystals, Cu(NO3)2,3H2O. This is a compound made up of copper, nitrogen, oxygen and hydrogen. These are present in the fixed proportions shown in the formula. Now imagine what a mixture of copper, nitrogen, oxygen and hydrogen would look like. Pure copper is a pinkish-brown metal, and the rest are all colourless gases: that bears no resemblance to the compound in the picture. And if you had this mixture, it could be any proportions. It would, of course, be trivial to get the copper out of the mixture - you could just pick it up! And although this isn't really feasible in a school lab, you could separate out all the gases just by cooling them enough. Oxygen turns to a liquid at -183°C and nitrogen becomes liquid at -196°C. That would just leave the hydrogen as a gas which doesn't liquefy until -253°C. This is basically how the individual gases that make up air are separated. Trying to get all the individual elements out of copper(II) nitrate crystals would be a bit of a nightmare! At the beginning of the page I suggested how you might turn copper(II) nitrate eventually into copper. Some of the oxygen present is given off as a gas when you heat the crystals, but the rest is still attached to the nitrogen (as nitrogen dioxide) and the hydrogen (as steam). Separating all of these elements would take several further chemical reactions. Separating mixtures can usually be done by physical means - heating, cooling, dissolving in water or some other solvent, using a magnet if one of them is magnetic, and so on. Once the elements are combined in a compound, you can only separate them by doing chemical reactions. A mixture of hydrogen and oxygen compared with its compound water, H2O The mixture, of course, could be present in any proportions, and would just be seen as a colourless gas. The water is a colourless liquid, and its molecules always contains two hydrogen atoms and one oxygen atom. You could separate the mixture of hydrogen and oxygen as described above by cooling it enough. But you can only separate the water into hydrogen and oxygen chemically - in this case, using electrolysis which we will look at in detail quite a lot further into the course. And the chemical properties of the mixture are totally different from the compound. Imagine putting a flame into a mixture of hydrogen and oxygen - you get a sizeable explosion as they react to make water. Put a flame into water, and it just goes out. There is a wonderful bit of video on YouTube showing what happens if you ignite a large balloon containing a hydrogen-oxygen mixture in exactly the right proportions.
© Jim Clark 2018 |
|