|
Chemguide: Core Chemistry 14 - 16 How to write formulae for simple ionic compounds This page explains how to work out the formulae of the simple ionic compounds that you will meet at this level. It is essential that you take our time over this, and don't leave the topic until you feel reasonably competent at writing these formulae. This is a key bit of chemistry, and the truth is that if you can't be bothered to do it properly, you might as well give up chemistry here and now. You can't succeed without this most basic chemistry tool. You will need to know about ionic bonding and have access to a Periodic Table such as the one you can download from this site. The download button is at the beginning of the second paragraph under the table. How writing formulae for ionic compounds works There are potentially thousands of ionic compounds whose formulae you could possibly be asked to write or recognise - although a relatively small number will turn up again and again. Trying to learn all of them would be a ridiculous waste of time, and really difficult. You will need to do a tiny bit of learning, but once that is done, the process is easy.
First of all, of course, you need to know that the compound is ionic. There is a simple bit of guidance to help you there, and I will give you that in a minute. Secondly, all these ionic compounds are overall electrically neutral. There have to be equal numbers of positive and negative charges in the compound. Knowing what those charges are is going to take up quite a lot of the rest of this page. There are a few simple generalisations, and a small amount of learning. Suppose, then, that you wanted to write the formula for the ionic compound, magnesium chloride. Suppose you knew that magnesium ions had a 2+ charge, Mg2+, and chloride ions had a 1- charge, Cl-. The magnesium chloride has to be electrically neutral overall. To balance the charges you would need 2 chloride ions for every magnesium ion: 2 negative charges to balance the 2 positive charges. So the formula for magnesium chloride is MgCl2. As another example, if you knew that the charge on a sodium ion was +1, Na+, and the charge on an oxide ion was 2-, O2-, then it is easy to see that the formula for sodium oxide is Na2O. You need to have two sodium ions to balance the charges on the oxide ion. Important! Notice the little numbers in these formulae. A number written smaller and subscripted (set lower) in a formula counts the number of atoms or ions immediately before it. So in the first formula, MgCl2, there are 2 chlorines, but only 1 magnesium - the 2 doesn't apply to the magnesium as well. In the second formula, Na2O, the 2 only applies to the sodium. Incidentally, the number 1 is never written into a formula - if there is no number after a symbol, then there is one of it. Once you know the charges on the ions making up the compound, working out the formula is easy. Setting the rules Some of these rules have exceptions, but you won't meet them at this introductory level - so we will ignore the exceptions for now. How do you know if a compound is ionic? A compound will be ionic if . . .
How do you know if an ion is positive or negative?
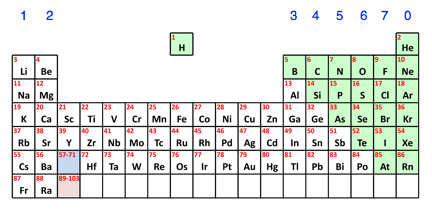
How do you know if something is a metal or non-metal? There is a simple pattern from the Periodic Table. Here is a simplified version of the Table missing out the bits that aren't relevant to this level. The non-metals are shown in green.
You can see that the non-metals are all found on the right-hand side of the Periodic Table. All the rest of the elements are metals. | |||||||||||||||||||||||||
|
Note: The dividing line between metals and non-metals isn't quite as clear-cut as this. Some of the elements just to the left of the green bit tend to have properties which are a mixture of metal and non-metal. This isn't something you need to worry about. The elements you will come across during a course at this level are clearly one or the other. | |||||||||||||||||||||||||
|
How do you name non-metal ions? Simple ions from non-metals have their endings changed to "ide". So the ions are called nitride, oxide, sulfide, fluoride, chloride, bromide, and iodide. Not all of the non-metals form simple ions. The ones mentioned above are the only simple ions you are likely to come across. Phosphorus does form a phosphide ion, but it isn't common. | |||||||||||||||||||||||||
|
Note: Are there non-simple ions? Yes! "Complex ions" are also common in the non-metals. These contain the non-metal plus other things as well - often oxygen. We will have more to say about this below. | |||||||||||||||||||||||||
|
How do you know how many charges an ion has? Where the name of the compound tells you the number of charges on a metal ion Quite a lot of compound names have a Roman numeral as a part of the name. (The proper term for this number is the oxidation state or oxidation number of the element. That won't concern you until you go on to do chemistry at a higher level.) For example . . .
This tells you how many positive charges the ion has. We are talking about the charges on a metal ion, and so they will always be positive. So . . .
Where you can work out the number of charges on a metal ion from a Periodic Table Let's look again at the Periodic Table from earlier on.
The numbers at the top of the Groups (the vertical columns in the table) are the old group numbers - from 1 to 7 and then 0. These ignore the transition metals. This has the big advantage for students at this level that it counts the number of electrons in the outer energy level of the atoms of each element. | |||||||||||||||||||||||||
|
Note: The last Group in the Periodic Table (the Noble Gases) is usually called Group 0, but was sometimes called Group 8. Helium, of course, only has room in its outer level for 2 electrons. | |||||||||||||||||||||||||
|
Modern numbering includes the transition metals, and the numbers go from 1 to 18. You will find this on the Periodic Table I suggested at the top of the page. To convert the new numbering to the old one, just subtract 10 from groups 13 to 17. Why does this matter? For the metals in Groups,1 2 and 3 (on the old numbering) the number of charges on the ions is the same as the Group number. That is because they have 1, 2 or 3 electrons in their outer levels to give away to something else. So. . .
The positive ions you will need to learn Group 1, 2 and 3 metals are easy, and so are all those with the Roman numerals in their name. There are a couple of metals, though, where the Roman numerals are left out more often than not. You will need to learn . . .
Don't forget that you will also need to know about these positive ions which don't contain metals . . .
Working out the charges on non-metal ions using the Periodic Table We are talking here about simple non-metal ions. The ones you need to know are oxide, sulfide, fluoride, chloride, bromide, and iodide in Groups 6 and 7. Elements at the top of Group 5 (nitrogen and phosphorus) also form negative ions in rare cases - so you should know how to work out the charges on nitride and phosphide ions as well just in case.
Elements in Groups 5, 6 and 7 have 5, 6 and 7 electrons in their outer energy levels. That means they have room to gain 3, 2 or 1 electrons to form ions with 8 electrons in the outer level.
| |||||||||||||||||||||||||
|
Note: For Group 5, this only really applies to nitrogen and to an even smaller extent phosphorus. Most of time, nitrogen and phosphorus form covalent bonds, or are a part of a complex ion (see below for one with nitrogen in). | |||||||||||||||||||||||||
|
Awkward complex negative ions A complex ion is one made up of more than one sort of atom.
You just have to learn these. Sorry! You may come across one or two more during the course, but these are enough to get started with. Some worked examples You need to have a copy of the Periodic Table available which has the names of the elements as well as their symbols. As you read through these examples, try to work them out for yourself before you read on. The more you pactise this, the easier it will become. What is the formula for chromium(III) chloride? First, you need the symbol for chromium. If you don't know it, find it! The name tells you that it has 3+ charges. By this time, you will probably know that the symbol for chlorine is Cl. It is in Group 7 and so has 7 outer electrons. There is room for 1 more in the outer level, and so a chloride ion has one extra negative electron. The ions are therefore Cr3+ and Cl-. To balance the charges on the chromium you will need 3 chloride ions. The formula is CrCl3. What is the formula for potassium sulfate? First, you need the symbol for potassium. If you don't know it, find it! Potassium is in Group 1 and so forms a 1+ ion. Sulfate is an ion you can't work out - you have to remember it. Go back up this page and look it up for now. The ions are therefore K+ and SO42-. To balance the charges on the sulfate ion you will need 2 potassium ions. The formula is K2SO4. What is the formula for iron(III) oxide? You need the symbol for iron. The name tells you that it has 3+ charges. The symbol for oxygen is O. It is in Group 6 and so has room for 2 more electrons in the outer level - so an oxide ion has 2 negative charges. The ions are therefore Fe3+ and O2-. This is slightly more tricky! The only way to balance the charges is to have 2 iron(III) ions and 3 oxide ions: 6 pluses and 6 negatives. The formula is Fe2O3. What is the formula for magnesium nitrate? Notice this is magnesium nitrate, NOT magnesium nitride - the ends of the words really matter. The nitrate ion is an ion which you will have to learn. The nitrate ion is NO3-. Magnesium is in Group 2 and so has 2+ charges, Mg2+. To balance the charges, you will need two nitrate ions for every magnesium ion. The formula is Mg(NO3)2. This is important! Notice the brackets around the nitrate group. If you need to have more than one complex ion, you must enclose it in brackets before you write any number after it. The "2" in the magnesium nitrate formula has to apply to the whole of the nitrate group. The only way of showing that is to enclose it in brackets. However, if you only need one of the group, you must not put brackets around it - that would be wrong. See, for example, the formula for potassium sulfate above. There are no brackets in this, because there is only one sulfate group. Some problems for you to do You will need a copy of the Periodic Table to refer to which must include the names of the elements. I suggest that you also make a list of those ions that need to be learnt on a piece of paper so that you can refer to them quickly. But do the problems first, and worry about learning them later. The fact that you keep will keep writing them down will mean that by the time you have finished this, you will probably have remembered most of them anyway. I have listed the problems in sets, and after each set, you will find a link to the answers for that set. Do one set at a time, and don't go on until you are sure that you completely understand any formula that you might have got wrong. Don't worry if it takes you a long time to start with - that is entirely to be expected. You will find it speeds up considerably once you get to know your way around the Periodic Table and the ions get more familiar. Once you have got to the end of this successfully, you will have cracked one of the big problems students find in the early stages of chemistry courses. To save you searching the whole Periodic Table for symbols, the metals used in the examples will mainly be from Groups 1, 2 and 3 (only aluminium in Group 3) or from the top row of the transition elements - but there will be a couple of other examples as well. Set 1 potassium iodide, iron(II) sulfide, calcium chloride, magnesium carbonate, lithium oxide Set 2 sodium bromide, copper(II) sulfate, barium hydroxide, nickel(II) chloride, lithium nitrate Set 3 aluminium chloride, lithium nitride, sodium nitrate, copper(II) nitrate, zinc sulfide Set 4 potassium carbonate, aluminium oxide, silver iodide, lead(II) nitrate, aluminium hydroxide Set 5 barium sulfate, zinc nitrate, ammonium chloride, sulfuric acid ("hydrogen sulfate"), lead(II) sulfide Set 6 ammonium sulfate, lead(II) bromide, nitric acid ("hydrogen nitrate"), chromium(III) sulfate, iron(III) hydroxide What now? If you made more than the occasional mistake, wait a day, re-read this page, and then do the problems again. It is essential that you don't leave this page until you can write formulae successfully. It doesn't matter if you haven't learnt all the awkward ions like nitrate or ammonium or silver at the moment. What matters is that you can put together a formula, even if you need to look some things up. If you have been successful most or all of the time, learn any of the ions which you don't know properly. You will find a complete list of the awkward ones below.
You will get more practice at writing formulae when we go on to look at writing equations.
© Jim Clark 2019 |
|||||||||||||||||||||||||