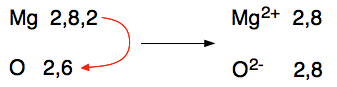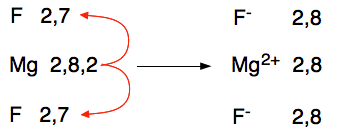|
Chemguide: Core Chemistry 14 - 16 Ionic (electrovalent) bonding This page shows how some atoms can bond by transferring electrons from one atom to another to produce electrically charged ions. These ions are strongly attracted to each other in ionic (sometimes called electrovalent) bonds. I am assuming that you have read the page on electronegativity and polar bonds and have access to a Periodic Table such as the one you can download from this site. The download button is at the beginning of the second paragraph under the table. Why do some bonds involve a transfer of electrons from one atom to another? If you have read the page about electronegativity and polar bonds, you will remember these diagrams. In a bond between two identical atoms, the bonding pair of electrons is found on average half-way between the two nuclei.
On the other hand, in a covalent bond between, say, carbon and fluorine, the bonding pair is pulled towards the fluorine atom, and the bond becomes electrically slightly distorted.
We say that fluorine is more electronegative than carbon. Electronegativity is a measure of an atom's ability to attract a bonding pair of electrons. The reason this happens is that there is a greater pull from the fluorine end because there are more protons in the fluorine nucleus, and identical shielding (screening). | |
|
Note: If you aren't sure about this, go and read the page on electronegativity and polar bonds again! You won't make sense of what comes next if you don't have a reasonable idea about this. | |
|
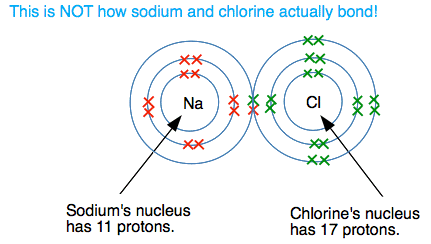
What happens if there is a large electronegativity difference between the two atoms? The classic example of this is sodium chloride (common salt). Let's suppose for a moment that the sodium formed a covalent bond with the chlorine. (It DOESN'T - this is just a starting point for what really happens!). A dots-and-crosses diagram for this would look like this:
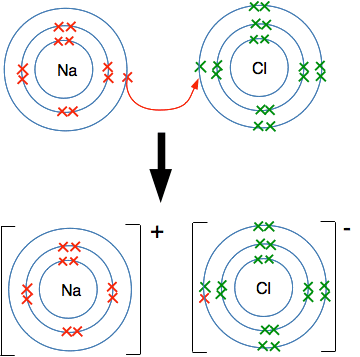
But this doesn't happen. Look at the different attractions from both ends of the bond. The sodium atom has 11 protons, but there are also 10 inner electrons cutting down the effect of these. There is roughly a net positive pull from the nucleus of 1+ (the charge on the 11 protons minus the effect of the 10 inner electrons). At the chlorine end, you can work out that there is a net pull of 7+ (the 17 protons less the effect of the 10 inner electrons). There is therefore a very strong attraction of the bonding electrons to the chlorine end of the bond - so strong that the sodium loses control of its outer electron. Essentially there is a transfer of the outer electron from the sodium to the chlorine. Because the sodium has lost an electron, it now carries a positive charge - it still has 11 protons, but now only has 10 electrons. It has formed a sodium ion, Na+. The chlorine has now gained an extra electron and has therefore gained a negative charge. It is now a chloride ion, Cl-. Missing out all the electrons not directly involved in this, you have:
Ways of showing this happening Using dots-and-crosses diagrams We can show the transfer of the electron like this:
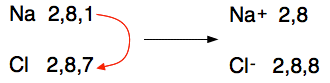
If you are asked to do it like this in an exam, then you have no choice. Otherwise it is a bit of a bother, and a bit more difficult to understand at a glance than using a much simpler method. Using the electronic structures If we just write the sodium as 2,8,1 and the chlorine as 2,8,7, then that is all the important information you need. Set it out like this:
What is an ionic bond? The powerful attraction between positive and negative ions is what makes an ionic bond. Large amounts of energy are released when the ions come together. But it is important to realise that it isn't only one sodium ion and one chloride ion which make up the bond - huge numbers of ions come together in a regular pattern called a lattice. The photo shows a tiny, tiny part of such a structure, and the pattern of alternating sodium ions (grey) and chloride ions (green) in all three dimensions just goes on and on until you get to the edge of the crystal.
This is known as a giant ionic structure. "Giant" in this context means more than just very, very big - it means that the size isn't defined. If you broke a crystal into two parts you would have two bits of this giant structure. That is different from the molecules you have seen when covalent bonds are formed. If you broke a large covalent molecule into two bits, you won't have the same substance any more. A covalent molecule is defined by a particular number and arrangement of atoms within it. So if you broke a large protein molecule, for example, into two bits, you don't have two smaller versions of the same molecule - you have wrecked it! If you break a salt crystal into smaller bits, you just have a whole lot of smaller salt crystals - all containing the same pattern of sodium and chloride ions. | |
|
Note: You will find later on that it is possible to have giant covalent structures where there are undefined numbers of atoms joined together with covalent bonds. Two examples of this are diamond (a 3-dimensional structure of carbon atoms joined by covalent bonds) and silicon dioxide (a 3-dimensional structure of silicon and oxygen joined by covalent bonds). | |
|
More examples of ionic bonds Magnesium oxide
This time the magnesium loses its two outer electrons to the oxygen to produce magnesium and oxide ions. There is a very strong ionic bond between the two because of the attraction between the 2+ and 2- ions. The arrangement of the ions is exactly the same as sodium chloride's. You can see this strength in the size of the melting point - that's how much you have to heat it to separate the ions and form a liquid. Magnesium oxide melts at 2852°C whereas sodium chloride melts at 801°C. The ionic bond in magnesium oxide is significantly stronger than in sodium chloride. Magnesium fluoride Magnesium's electronic structure is 2,8,2, and fluorine's 2,7. When the transfer of electrons takes place, to have enough room to accept the magnesium's 2 outer electrons needs two fluorine atoms.
The chemical formula for magnesium fluoride is MgF2, reflecting what has happened in the bonding. Because you now have twice as many fluoride ions as magnesium ions, the lattice structure of magnesium fluoride must be different from the last two cases. Unlike the sodium chloride case which you may well be asked to describe or draw, complex structures like magnesium fluoride are well beyond this level. We will look at how you draw a sodium chloride lattice on another page. This is another strong ionic bond, although not as strong as the magnesium oxide case where you have 2+ attracting 2- ions. Lithium oxide The reverse happens here - you need two lithium atoms to provide the electrons for the oxygen.
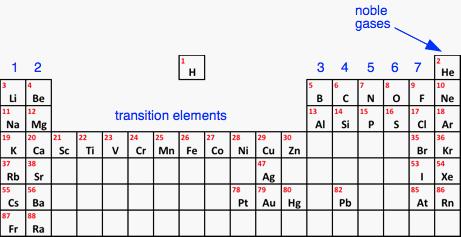
Again, there is a strong ionic bond between the lithium and the oxide ions. The chemical formula for lithium oxide is Li2O. What decides how many electrons get transferred? The rest of this page goes more deeply into the topic than is usual at this level. I am doing it because simplifications taught now can cause problems later on. I don't think it is very difficult and I have added simple summaries of what you need to know as we go along. When atoms form ions, there are a lot of different energy processes involved which are beyond this level. But the most important factors are the amount of energy it takes to form the positive ions, and the amount of energy released when the ions come together. The higher the charges on the ions formed, the more energy is released when the ions come together. But the more highly charged the ions, the more energy it takes to make them in the first place. These two effects are in direct competition. The Periodic Table You may remember this simplified Periodic Table from previous pages which leaves out most of the elements you will never meet at this level.
The numbers in blue are the (old) group numbers. I am using the old numbers because they have the advantage of telling you the number of electrons in the outer level of each of the elements in that group. The transition elements are all metals - in fact, they are often called transition metals. The group on the far right of the table is important in this work and is usually known as the Noble Gases. Formation of negative ions Simple negative ions are made by elements in Group 7, and also the elements towards the top of Groups 6 and 5.
In each of those cases, the original atom has gained either 1 or 2 or 3 electrons from something else to make the negative ion. And in each case, the electronic structure of the ion created is the same as the noble gas at the end of the period. So, for example:
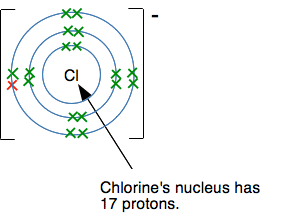
Why does this happen? Why doesn't chlorine, for example, form a 2- ion with the structure 2,8,8,1? The answer is often given that the elements produce what are called noble gas structures when they form ions because these are stable. But that begs the question of why they are stable. It is interesting to look at what would happen if a chloride ion were to try to accept another electron to make a Cl2- ion. This is what a chloride ion's electronic structure looks like:
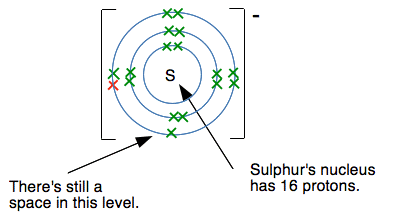
Another electron would have to go into a level further from the nucleus, and the nucleus would be screened from the new electron by all the electrons currently present. Count them! There are 17 positive protons in the nucleus, and currently 18 negative electrons surrounding it. There is a net 1- charge on the ion. There is no way that another electron would attach itself to this - it would be repelled by the combined effect of the nucleus and inner electrons. So why does sulfur form a 2- ion? Doesn't the same thing apply? This diagram shows what the sulfur's electronic structure is after it has gained one electron to make an S- ion.
But this time, there is still room at the 3-level for another electron. If another electron fills that space, it feels the pull of the 16 protons offset by the inner 10 electrons. In other words, there is still a 6+ pull from the centre of the ion. The fact that there is an extra electron in the 3-level doesn't affect the screening. Creating the 2- ion means that the attractions between this ion and whatever positive ion you have attached to it will be maximised. Summary for the formation of simple negative ions
Formation of positive ions from Groups 1,2 and 3 Boron at the top of Group 3 doesn't form positive ions, so that is excluded from what follows. | |
|
Note: Beryllium at the top of Group 2 is also odd. It doesn't form simple Be2+ ions in the solid state, but does form ions in solution, wrapped up in water molecules - for example, Be(H2O)42+. You don't need to know this! Ignore beryllium! | |
|
These elements (apart from boron) are all metals and lose all of the electrons in their outer level when they form ions. All metals form positive ions. For example:
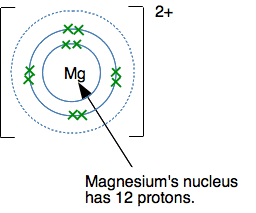
In each case, the ion formed has a noble gas structure. I have just taken the first three elements from the period starting with sodium, but it would be equally true for any other element in these groups. For example, Ca (2,8,8,2) forms a Ca2+ ion (2,8,8) by losing its outer electrons. That has the same electronic structure as the noble gas argon. Why does magnesium form a 2+ ion? Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the extra energy released when a 2+ ion is attracted to whatever negative ion is formed as well. But once you have removed both of the outer electrons, the magnesium electronic structure looks like this:
If you try to remove another electron, it is coming out of a level closer to the nucleus, and screened from the nucleus by only 2 inner electrons. The outer electrons are now feeling a pull of about 10+ from the centre (the 12 protons minus the two inner electrons). That would require a huge amount of energy to pull another electron away from the magnesium! And so it doesn't happen. The same thing is true for all of these metals in Groups 1, 2 and 3. You can't remove electrons from the inner levels because it would require too much energy. Summary for the formation of positive ions in Groups 1, 2 and 3
Formation of positive ions from transition and other metals If you look at a full copy of the Periodic Table, you will see that there are far more metals in this middle block of the table than there are in Groups 1, 2 and 3. There are also some metals, like lead, to the right of the transition metals in the Periodic Table. Apart from a few metals at the extreme left-hand side of the transition metals (such as scandium, Sc, for example), none of the rest form ions with noble gas structures. Here are a few common examples - none of which you will be expected to work out or remember.
Summary for the formation of positive ions in other metals The vast majority of metal ions elsewhere in the Periodic Table do not have noble gas structures.
© Jim Clark 2019 |
|