|
Chemguide: Core Chemistry 14 - 16 How to write formulae for simple covalent substances This page explains how to work out the formulae of the simple covalent substances that you will meet at this level. For the most part, this isn't a problem. You will quickly become familiar with the common covalent substances, and will just get to know the formulae. You will need to know about covalent bonding and have access to a Periodic Table such as the one you can download from this site. The download button is at the beginning of the second paragraph under the table. This is unlikely to be a problem! There aren't that many covalently bound substances that you will meet in the early stages of a chemistry course, and you will come across most of them frequently enough that you will eventually just know them. For example, few people will have to work out the formula of water as being H2O. It is in common use. You may have come across the little poem:
H2SO4 is sulfuric acid. Concentrated sulfuric acid is a rather oily covalent liquid - although it does form ions in the presence of water. Most people never work out the formula for sulfuric acid - they just know it. And there are a whole lot of cases where the name tells you. It wouldn't be too hard to write the formula for carbon dioxide, or carbon monoxide, or sulfur dioxide, or nitrogen dioxide, or phosphorus trichloride - although in the very early stages you might need a Periodic Table to check on the symbols of the elements concerned. These compounds are CO2, CO, SO2, NO2 and PCl3. "mono" implies 1; "di" implies 2; "tri" implies 3. There are also several non-metallic elements which go around in covalently bound pairs - so called diatomic molecules. So, for example, ordinary oxygen gas is O2, and not just O. Other common examples are H2, N2, F2, Cl2, Br2 and I2. You will meet most of these so often that it will become second nature to write them down without thinking about them. | |
|
Note: Don't go away with the idea that all non-metallic elements go around in pairs - they don't! The noble gases at the extreme right of the Periodic Table occur as single atoms, and other a few other non-metals have molecules with more than two atoms. The ones I have listed are the common ones that you will meet frequently. | |
|
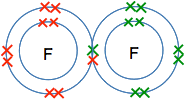
But suppose you do have to work something out? The diatomic molecules Why is the formula for fluorine F2? Let's start by sorting out the difference between the symbol for fluorine and the formula of fluorine. The symbol for fluorine is what you find on the Periodic Table, F, and refers to a single atom of fluorine. The formula for fluorine refers to the way it is found under normal circumstances - that turns out to be F2. If you work out the electronic structure of fluorine, you will find that it is 2,7. If you draw a dots-and-crosses picture of this, you will find that it has a single unpaired electron. If it could use that electron to form a covalent bond it would release energy and become energetically more stable. So it shares an electron with another fluorine . . .
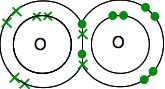
. . . and so forms a molecule, F2. Why is the formula for oxygen O2? If you go through this whole process again with oxygen, you will find that it has an electronic structure of 2,6 with two unpaired electrons. In this case, it can share each of these electrons with electrons from another oxygen atom to give a molecule O2.
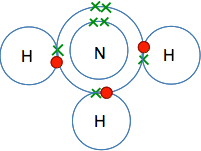
This is all a bit tedious, of course, if all you need to know is what formula to write down in a chemical equation. Nobody in their right minds is ever going to do this in real life! Once you have written the formula for hydrogen, H2, or chlorine, Cl2, a few times you will just do it without thinking about it. You might, though, be asked in an exam to explain why the formula for chlorine is Cl2, and so you need to be able to do it, even if you never actually do it in real life. A couple of other molecules Ammonia You won't meet ammonia as often as things like hydrogen, oxygen and chlorine, and eventually you will probably just learn the formula. If you had to work it out, this is how it would go . . . You would need to know that ammonia was a simple compound of nitrogen and hydrogen. You would use the Periodic Table to work out that the electronic structure of nitrogen was 2,5. If you drew that in a dots-and-crosses diagram, you would have 3 single electrons and 1 pair in the outer level. It would be most energetically stable if all of those single electrons were used to make covalent bonds. If you do the same thing with hydrogen, you will find it has 1 electron in its only layer. To satisfy both the nitrogen and the hydrogens you could make three covalent bonds:
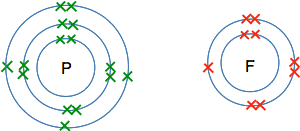
So the formula for ammonia is NH3. The simplest compound of phosphorus and fluorine This will never be a familiar compound to you - so you would have to work it out if you ever needed it. If you worked out the electronic structure of both phosphorus and fluorine you would get:
To get all the single electrons paired, you would need 3 fluorine atoms for each phosphorus atom. There is no need to draw the whole thing - just imagine it. So the formula for the compound is PF3.
© Jim Clark 2019 |
|