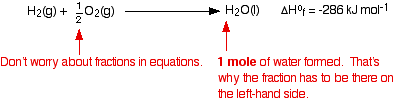|
VARIOUS ENTHALPY CHANGE DEFINITIONS This page explains what an enthalpy change is, and then gives a definition and brief comment for three of the various kinds of enthalpy change that you will come across. You will find some more definitions on other pages in this section. It is essential that you learn the definitions. You aren't going to be able to do any calculations successfully if you don't know exactly what all the terms mean. Enthalpy changes Enthalpy change is the name given to the amount of heat evolved or absorbed in a reaction carried out at constant pressure. It is given the symbol ΔH, read as "delta H". | |||
|
Note: The term "enthalpy change" only applies to reactions done at constant pressure. That is actually how most lab reactions are done - in tubes or flasks (or whatever) open to the atmosphere, so that the pressure is constant at atmospheric pressure. The phrase "at constant pressure" is an essential part of the definition but, apart from that, you are unlikely to need to worry about it if you are doing a UK-based exam at the equivalent of A level. | |||
|
Standard enthalpy changes Standard enthalpy changes refer to reactions done under standard conditions, and with everything present in their standard states. Standard states are sometimes referred to as "reference states". Standard conditions Standard conditions are:
| |||
|
Warning! Standard pressure was originally defined as 1 atmosphere (101.325 kPa), and you will still find that in older books (including my calculations book). At the time of writing (August 2010) there was at least one UK-based syllabus that was still talking in terms of "1 atmosphere". It is essential to check your syllabus to find out exactly what you need to learn. | |||
|
Standard states For a standard enthalpy change everything has to be present in its standard state. That is the physical and chemical state that you would expect to find it in under standard conditions. That means that the standard state for water, for example, is liquid water, H2O(l) - not steam or water vapour or ice. Oxygen's standard state is the gas, O2(g) - not liquid oxygen or oxygen atoms. For elements which have allotropes (two different forms of the element in the same physical state), the standard state is the most energetically stable of the allotropes. For example, carbon exists in the solid state as both diamond and graphite. Graphite is energetically slightly more stable than diamond, and so graphite is taken as the standard state of carbon. Similarly, under standard conditions, oxygen can exist as O2 (simply called oxygen) or as O3 (called ozone - but it is just an allotrope of oxygen). The O2 form is far more energetically stable than O3, so the standard state for oxygen is the common O2(g). The symbol for standard enthalpy changes The symbol for a standard enthalpy change is ΔH°, read as "delta H standard" or, perhaps more commonly, as "delta H nought". | |||
|
Note: Technically, the "o" in the symbol should have a horizontal line through it, extending out at each side. This is such a bother to produce convincingly without the risk of different computers producing unreliable results, that I shall use the common practice of simplifying it to "o". | |||
|
Standard enthalpy change of reaction, ΔH°r Remember that an enthalpy change is the heat evolved or absorbed when a reaction takes place at constant pressure.
That needs exploring a bit. Here is a simple reaction between hydrogen and oxygen to make water:
Some important types of enthalpy change Standard enthalpy change of formation, ΔH°f
| |||
|
Note: When you are trying to learn these definitions, you can make life easier for yourself by picking out the key bit, and adding the other bits on afterwards. The key bit about this definition is that you are forming 1 mole of a compound from its elements. All the stuff about enthalpy change and standard conditions and standard states is common to most of these definitions. | |||
|
The equation showing the standard enthalpy change of formation for water is:
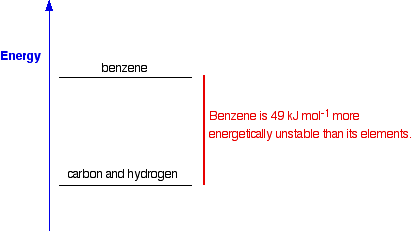
When you are writing one of these equations for enthalpy change of formation, you must end up with 1 mole of the compound. If that needs you to write fractions on the left-hand side of the equation, that is OK. (In fact, it is not just OK, it is essential, because otherwise you will end up with more than 1 mole of compound, or else the equation won't balance!) The equation shows that 286 kJ of heat energy is given out when 1 mole of liquid water is formed from its elements under standard conditions. Standard enthalpy changes of formation can be written for any compound, even if you can't make it directly from the elements. For example, the standard enthalpy change of formation for liquid benzene is +49 kJ mol-1. The equation is:
If carbon won't react with hydrogen to make benzene, what is the point of this, and how does anybody know what the enthalpy change is? What the figure of +49 shows is the relative positions of benzene and its elements on an energy diagram:
How do we know this if the reaction doesn't happen? It is actually very simple to calculate it from other values which we can measure - for example, from enthalpy changes of combustion (coming up next). We will come back to this again when we look at calculations on another page. Knowing the enthalpy changes of formation of compounds enables you to calculate the enthalpy changes in a whole host of reactions and, again, we will explore that in a bit more detail on another page. And one final comment about enthalpy changes of formation: The standard enthalpy change of formation of an element in its standard state is zero. That's an important fact. The reason is obvious . . . For example, if you "make" one mole of hydrogen gas starting from one mole of hydrogen gas you aren't changing it in any way, so you wouldn't expect any enthalpy change. That is equally true of any other element. The enthalpy change of formation of any element has to be zero because of the way enthalpy change of formation is defined. | |||
|
Note: Some sources say that the enthalpy change of formation of elements is taken as zero by convention. That is simply nonsense! The standard enthalpy change of formation of elements is zero because of the way the enthalpy change is defined. If this confuses you, ignore it! | |||
|
Standard enthalpy change of combustion, ΔH°c
The enthalpy change of combustion will always have a negative value, of course, because burning always releases heat. Two examples:
Notice:
To the chemical energetics menu . . . © Jim Clark 2010 (modified May 2013) |
|||