|
THE HYDROLYSIS OF PROTEINS This page looks briefly at the hydrolysis of proteins into their constituent amino acids using hydrochloric acid. Hydrolysing proteins using hydrochloric acid The chemistry of the reaction If you have already studied the hydrolysis of amides under acidic conditions, you will find that this is basically the same reaction. That's not surprising because what biologists and biochemists call a peptide link (in proteins, for example) is what chemists call an amide link. With an amide like ethanamide, the carbon-nitrogen bond in the amide group is broken and you get a carboxylic acid formed:
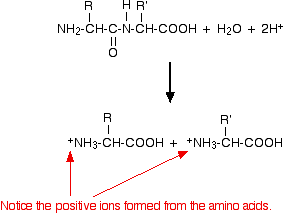
Now imagine doing the same thing with a simple dipeptide made of any two amino acids.
Instead of ammonium ions, you get positive ions made from the -NH2 groups reacting with hydrogen ions. You need the extra hydrogen ion in the equation (compared with the amide equation) to react with the -NH2 group on the left-hand end of the dipeptide - the one not involved in the peptide link. If you scale this up to a polypeptide (a protein chain), each of the peptide links will be broken in exactly the same way. That means that you will end up with a mixture of the amino acids that made up the protein - although in the form of their positive ions because of the presence of the hydrogen ions from the hydrochloric acid. | ||
|
Note: If you aren't sure about the formation of these positive ions from the reactions between amino acids and hydrogen ions, it would be useful to follow this link. Use the BACK button on your browser to return to this page. | ||
|
Doing the reaction There are two ways of carrying out this reaction - an old, slow method, and a new, fast one. The old slow way The protein is heated with 6 M hydrochloric acid for about 24 hours at 110°C. (6M hydrochloric acid is slightly more than semi-concentrated.) The new fast way Protein samples are placed in tubes in a sealed container containing 6 M hydrochloric acid in an atmosphere of nitrogen. The whole container is then placed in a microwave oven for about 5 - 30 minutes (depending on the protein) with temperatures up to 200°C. The hydrochloric acid vaporises, comes into contact with the protein samples and hydrolyses them. This method is used to hydrolyse small samples of protein during protein analysis.
© Jim Clark 2004 (modified May 2016) |
||