|
CARBOCATIONS (or CARBONIUM IONS) All carbocations (previously known as carbonium ions) carry a positive charge on a carbon atom. The name tells you that - a cation is a positive ion, and the "carbo" bit refers to a carbon atom. However there are important differences in the structures of various types of carbocations. The different kinds of carbocations Primary carbocations In a primary (1°) carbocation, the carbon which carries the positive charge is only attached to one other alkyl group. | ||
|
Help! An alkyl group is a group such as methyl, CH3, or ethyl, CH3CH2. These are groups containing chains of carbon atoms which may be branched. Alkyl groups are given the general symbol R. | ||
|
Some examples of primary carbocations include: Notice that it doesn't matter how complicated the attached alkyl group is. All you are doing is counting the number of bonds from the positive carbon to other carbon atoms. In all the above cases there is only one such link. Using the symbol R for an alkyl group, a primary carbocation would be written as in the box. Secondary carbocations In a secondary (2°) carbocation, the carbon with the positive charge is attached to two other alkyl groups, which may be the same or different. Examples: A secondary carbocation has the general formula shown in the box. R and R' represent alkyl groups which may be the same or different. Tertiary carbocations In a tertiary (3°) carbocation, the positive carbon atom is attached to three alkyl groups, which may be any combination of same or different. A tertiary carbocation has the general formula shown in the box. R, R' and R" are alkyl groups and may be the same or different. The stability of the various carbocations At the end of 2024, the Royal Society of Chemistry (RSC) emailed UK Exam Boards suggesting that the way this has been taught traditionally by way of an inductive effect of alkyl groups (often described as an "electron pushing effect") can be shown to be wrong. I'm going to give you the traditional version and the new approved version. Which should you use? The answer to this is always use whatever your examiners want - check your syllabus. I know that AQA have said that until they publish a new syllabus sometime in the future they will accept either version. It may well make sense to continue to use the traditional explanation until your syllabus changes because there will be enough evidence in past papers for exactly what they want you to say. Take the advice of your teachers. Whichever approach you use, the fact is that alkyl groups are electron-donating. They make whatever they are attached to slightly more negative or slightly less positive. So if you have a carbocation with one or more alkyl groups attached to the carbon carrying the positive charge, they will lower that charge. The difference between the traditional and more accurate approaches is trying to explain why that is. The traditional approach: the "electron pushing effect" of alkyl groups You are probably familiar with the idea that bromine is more electronegative than hydrogen, so that in a H-Br bond the electrons are held closer to the bromine than the hydrogen. A bromine atom attached to a carbon atom would have precisely the same effect - the electrons being pulled towards the bromine end of the bond. The bromine has a negative inductive effect. | ||
|
Help! If you aren't familiar with all of this, follow this link to read about electronegativity and bond polarity before you go any further. Use the BACK button on your browser to return to this page. | ||
|
Alkyl groups do precisely the opposite and, rather than draw electrons towards themselves, tend to "push" electrons away. | ||
|
Note: The term "electron pushing" is only to help remember what happens. There is a transfer of some negative charge towards to carbon with the positive charge on it. | ||
|
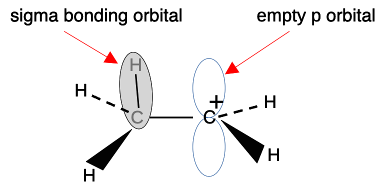
This means that the alkyl group becomes slightly positive ( This is sometimes shown as, for example: The arrow shows the electrons being "pushed" away from the CH3 group. The plus sign on the left-hand end of it shows that the CH3 group is becoming positive. The symbols If this is what your syllabus wants, don't bother with the next bit - you will just get confused. Instead, jump over it to the rest of the page by clicking here. The more accurate approach: hyperconjugation We'll look at a simple carbonium ion from the ones in the diagram above. Hyperconjugation looks at what is happening at the orbital level. I want to concentrate on the positive carbon atom and one of the carbon-hydrogen bonds, looking at the ion CH3CH2+.
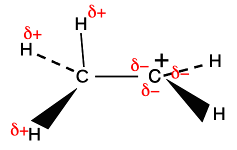
The sigma bond shown between the hydrogen and carbon is simply a pair of shared electrons. The empty orbital on the positive carbon is because the two electrons originally in that orbital were removed by something else. These ions only form as a short-lived stage of a reaction mechanism. Notice that the two orbitals shown aren't far off being parallel. And remember that these diagrams are shown in an "exploded" form. The orbitals actually touch to an extent - not enough to form a bond by sideways overlap, but enough to have some temporary interaction. Why "temporary"? Because there is free rotation around a single bond - the CH3 is constantly spinning around putting one after another C-H bond in the right position to interact with the empty p orbital. The hydrogens at the ends of the sigma bonds have a slight positive charge because on average the electrons in the bond have moved slightly towards the positively charged carbon. The positive nuclei are still there, but their charge is no longer quite covered by the bond electrons. At the same time, addition of some negative charge around the right-hand carbon is slightly cutting down its positive charge.
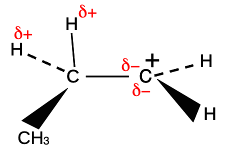
If there is more than one CH3 group attached to the positive carbon, this same thing is happening to all of them, leading to even more δ- charge accumulating around the positive carbon. Be careful if you have a more complicated alkyl group like ethyl, CH3CH2, bonded to the positive C. Only the two hydrogens on the carbon directly attached to the positive carbon can get involved in this. The others on the CH3 group are too far away.
| ||
|
Note: You may have noticed that I used the term "hyperconjugation" in the heading for this section and haven't mentioned it again. In the whole of my teaching experience, I have never used this term even once until now, and it is very unlikely if you will meet it again at this level.
If you you are at the beginning of a course at the 16-18 level and try to look up a definition of the word, you will probably regret it! Just take it as a long word which relates to what we have been talking about in this section.
| ||
|
The importance of spreading charge around in making ions stable The general rule-of-thumb is that if a charge is very localised (all concentrated on one atom) the ion is much less stable than if the charge is spread out over several atoms. Applying that to carbocations of various sorts . . . | ||
|
Note: I have simplified this slightly by just showing the the δ+ charges on the CH3 group as a whole, and only one δ- to represent all of the separate contributions from a particular CH3 group. It's just to simplify the diagrams, but also means that this section can be used by people using the traditional approach as well. | ||
|
You will see that the electron transfer from the CH3 group is placing more and more negative charge on the positive carbon as you go from primary to secondary to tertiary carbocations. The effect of this, of course, is to cut down that positive charge. At the same time, the region around the various CH3 groups is becoming somewhat positive. The net effect, then, is that the positive charge is being spread out over more and more atoms as you go from primary to secondary to tertiary ions. The more you can spread the charge around, the more stable the ion becomes.
| ||
|
Note: The symbol "<" means "is less than". So what this is saying is that primary ions are less stable than secondary ones which in turn are less stable than tertiary ones. | ||
|
The stability of the carbocations in terms of energetics When we talk about secondary carbocations being more stable than primary ones, what exactly do we mean? We are actually talking about energetic stability - secondary carbocations are lower down an energy "ladder" than primary ones. This means that it is going to take more energy to make a primary carbocation than a secondary one. If there is a choice between making a secondary ion or a primary one, it will be much easier to make the secondary one. Similarly, if there is a choice between making a tertiary ion or a secondary one, it will be easier to make the tertiary one. This has important implications in the reactions of unsymmetrical alkenes. If you are interested in these, follow the link below to the electrophilic addition reactions menu.
© Jim Clark 2000 (modified February 2025) |
||