|
THE SHAPES OF COMPLEX METAL IONS This page describes the shapes of some common complex metal ions. It goes on to look at some simple examples of stereoisomerism (geometric and optical) in complex ions. If you aren't sure what stereoisomerism is, you will find a helpful link further down the page. Some simple shapes for complex ions These shapes are for complex ions formed using monodentate ligands - ligands which only form one bond to the central metal ion. You will probably be familiar with working out the shapes of simple compounds using the electron pair repulsion theory. Unfortunately that doesn't work for most complex metal ions involving transition metals. The answer is just to learn the shapes you need to know about. As you will see, it isn't difficult. | ||
|
Note: If you are interested (although it is completely irrelevant to this page), you will find an explanation of how you work out the shapes of simple molecules and ions by following this link. You don't need to know about this to understand the rest of this page! | ||
|
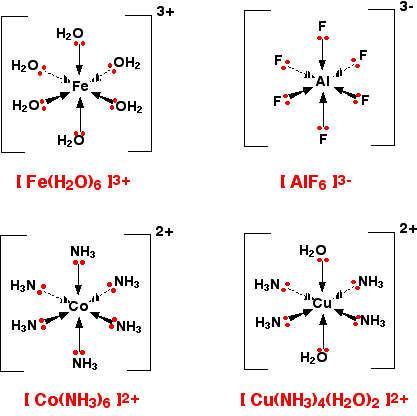
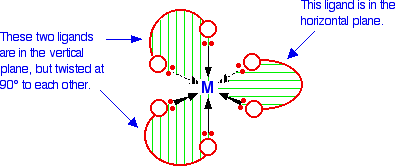
6-co-ordinated complex ions These are complex ions in which the central metal ion is forming six bonds. In the simple cases we are talking about, that means that it will be attached to six ligands. These ions have an octahedral shape. Four of the ligands are in one plane, with the fifth one above the plane, and the sixth one below the plane. The diagram shows four fairly random examples of octahedral ions.
| ||
|
Note: Remember that the ligands attached to a wedge shaped arrow are coming out of the screen or paper towards you. Those attached to a dotted arrow are behind the plane of the screen or paper. The two ligands attached to the ordinary arrows are above and below the plane of the rest. | ||
|
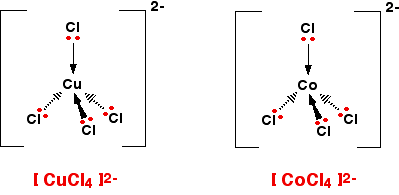
It doesn't matter what the ligands are. If you have six of them, this is the shape they will take up. Easy! 4-co-ordinated complex ions These are far less common, and they can take up one of two different shapes. Tetrahedral ions These are the ones you are most likely to need for A' level purposes in the UK. There are two very similar ions which crop up commonly at this level: [CuCl4]2- and [CoCl4]2-. The copper(II) and cobalt(II) ions have four chloride ions bonded to them rather than six, because the chloride ions are too big to fit any more around the central metal ion.
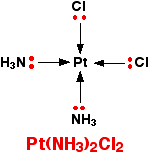
That's not very difficult to remember either! A square planar complex Occasionally a 4-co-ordinated complex turns out to be square planar. There's no easy way of predicting that this is going to happen. The only one you might possibly come across at this level is cisplatin which is used as an anti-cancer drug. Cisplatin is a neutral complex, Pt(NH3)2Cl2. It is neutral because the 2+ charge of the original platinum(II) ion is exactly cancelled by the two negative charges supplied by the chloride ions.
The platinum, the two chlorines, and the two nitrogens are all in the same plane. We will have more to say about cisplatin immediately below. Stereoisomerism in complex ions Some complex ions can show either optical or geometric isomerism. | ||
|
Warning! If you don't know about optical and geometric isomers, it is essential that you explore the isomerism menu in the organic chemistry section of this site before you go any further. The rest of this page is unlikely to mean anything to you if you don't have this background! Use the BACK button, or the History file, or the Go menu on your browser to return to this page - depending on how waylaid you get! | ||
|
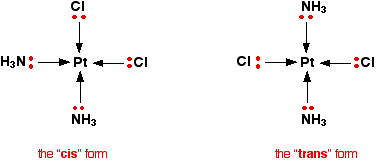
Geometric isomerism This occurs in planar complexes like the Pt(NH3)2Cl2 we've just looked at. There are two completely different ways in which the ammonias and chloride ions could arrange themselves around the central platinum ion:
The two structures drawn are isomers because there is no way that you can just twist one to turn it into the other. The complexes are both locked into their current forms. The terms cis and trans are used in the same way as they are in organic chemistry. Trans implies "opposite" - notice that the ammonias are arranged opposite each other in that version, and so are the chlorines. Cis implies "on the same side" - in this instance, that just means that the ammonias and the chlorines are next door to each other. Optical isomerism You recognise optical isomers because they have no plane of symmetry. In the organic case, it is fairly easy to recognise the possibiliy of this by looking for a carbon atom with four different things attached to it. It isn't quite so easy with the complex ions - either to draw or to visualise! The examples you are most likely to need occur in octahedral complexes which contain bidentate ligands - ions like [Ni(NH2CH2CH2NH2)3]2+ or [Cr(C2O4)3]3-. | ||
|
Help! If these ions look scarily unfamiliar, you must read the introductory page on complex ions before you go on. The shapes of the ions given below will be simplified in a way that is explained on that page. Use the BACK button on your browser to return to this page. | ||
|
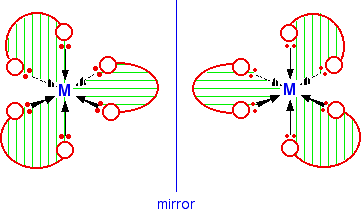
The diagram below shows a simplified view of one of these ions. Essentially, they all have the same shape - all that differs is the nature of the "headphones". I have deliberately left the charges off the ion, because obviously they will vary from case to case. The shape shown applies to any ion of this kind.
If your visual imagination will cope, you may be able to see that this ion has no plane of symmetry. If you find this difficult to visualise, the only solution is to make the ion out of a lump of plasticene (or a bit of clay or dough) and three bits of cardboard cut to shape. A substance with no plane of symmetry is going to have optical isomers - one of which is the mirror image of the other. One of the isomers will rotate the plane of polarisation of plane polarised light clockwise; the other rotates it anti-clockwise. | ||
|
Help! If you don't understand what I am talking about, it is because you didn't take my advice and follow the link to the isomerism part of the site earlier on this page! You need to read about optical isomerism in the organic chemistry section of this site. Use the BACK button on your browser to return to this page . | ||
|
In this case, the two isomers are:
If you have a really impressive visual imagination, you may be able to see that there is no way of rotating the second isomer in space so that it looks exactly the same as the first one. I can't do this! The only way I have ever been able to convince myself that they are different is to make models. For exam purposes, this doesn't matter in the slightest. As long as you draw the isomers carefully, with the second one a true reflection of the first, the two structures will be different.
© Jim Clark 2003 (modified November 2014) |
||