|
Chemguide: Core Chemistry 14 - 16 The reactions between metals and water or steam This page explores the way the position of a metal in the reactivity series affects its reaction with water or steam. The position of hydrogen in the reactivity series
We haven't so far mentioned the position of hydrogen in the reactivity series, but it is really important for this topic and the next. Water is H2O - hydrogen oxide. If a metal is above hydrogen in the reactivity series it should, in principle, be able to remove the oxygen from the water to leave hydrogen gas. Metals below hydrogen won't be able to react with water in this way. | |||||||||||||||||||||||||||||||||||||||||||
|
Note: I say "in principle" because sometimes a metal that you might expect to react with water or steam doesn't, or only reacts much more slowly than you might expect. There are a couple of cases like that, and we will talk about them individually. | |||||||||||||||||||||||||||||||||||||||||||
|
We will start by looking at the reactions of the metals at the top of the reactivity series. The reactions of potassium, sodium and lithium at the top of the series If you looked at a copy of the Periodic Table you would find these metals at the top of Group 1. As you will find later in the course, the reactivity of these metals increases the further down the group you go. So, in this bit of video from the Open University they are starting with lithium at the top of the group, and then working down. For reasons that will become obvious, you won't usually come across rubidium and caesium in the lab. In each case, you get hydrogen produced and a solution of the metal hydroxide.
For example
If you watch the video again, in the relatively peaceful reactions, you will see a white trail under the metals as they move around. This is the metal hydroxide formed dissolving in the water. | |||||||||||||||||||||||||||||||||||||||||||
|
Note: You may wonder why you get a metal hydroxide formed rather than an oxide. The oxides of these metals all react with water to produce a hydroxide and so that is what you end up with. This also applies to the reaction of calcium with water and to magnesium's very slight reaction with cold water (below). | |||||||||||||||||||||||||||||||||||||||||||
|
The reactions of calcium and magnesium with water Reactions with cold water Calcium's reaction with water is similar to lithium's. It looks a bit different because calcium is denser than water and so sinks. The first bit of video shows quite a large chunk of calcium being dropped into water. You can see bubbles of hydrogen being produced. The cloudiness is due to the formation of calcium hydroxide. This isn't very soluble in water and a lot of it is formed as a solid. You should also notice that the calcium moves around in the beaker, carried by the bubbles of hydrogen.
Magnesium and calcium are at the top of Group 2 of the Periodic Table, and there is also a very, very short piece of video comparing the reactions of water of magnesium, calcium, strontium and barium (two other member of Group 2). It just shows four test tubes with each of the metals in water. Concentrate on the first two tubes which contain magnesium and calcium. Notice that there doesn't seem to be any reaction with the magnesium. What happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble magnesium hydroxide, and that stops further water getting at the magnesium. If you put a small, but very clean, coil of magnesium in water and leave it for half-an-hour or so, bubbles of hydrogen do form on the surface and will often float the magnesium to the surface. Magnesium's reaction with steam Magnesium will, however, burn in steam to produce magnesium oxide and hydrogen. You get magnesium oxide produced rather than magnesium hydroxide at these higher temperatures. That also applies to the other metals that we will need to react with steam. The next bit of video shows a boiling tube with a coil of magnesium ribbon in it. At the bottom of the tube is a wad of mineral wool soaked in water. The magnesium is heated until it just starts to react, and then the heat is moved to produce a lot of steam.
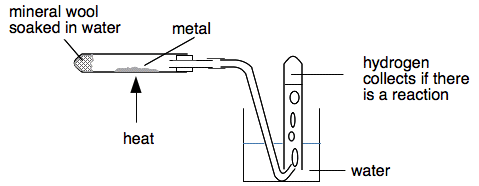
You would expect white magnesium oxide to be formed, but the tube usually shows a lot of black product as well as white. This bit of video avoids this problem by stopping it before you get to notice this! What happens is that the magnesium reacts with the glass as well as the steam. The black product is a mixture of boron and silicon - you don't need to remember this! Burning magnesium's reaction with water I came across this reaction which I had never seen before (and would never have thought of trying) while I was searching for good videos for this page. It comes from a wonderful collection of short videos about the elements in the Periodic Table from the University of Nottingham. They are presented by Sir Martyn Poliakoff who is a research professor at the university. I offer this just as a bonus - not for you to remember! Reactions of the other metals in the Reactivity Series If they are going to react at all, these metals have to be heated strongly in steam. In a school lab, a common bit of apparatus is:
The metal is heated strongly, and heat leaks back along the tube and turns the water in the mineral wool to steam. You might occasionally move the Bunsen back under the mineral wool for a few seconds to increase the steam flow. It is important to realise that just getting some bubbles in the collecting tube isn't enough to show that hydrogen is produced. The steam will force all the air that was originally in the tube over into the collecting tube. That means that you are bound to collect nearly a test tube full of gas whether there is a reaction or not. But if you collect more than a test tube of gas, then there must be hydrogen being produced. And, of course, you could test it with a lighted splint when it will give a small pop. At the end of the experiment, it is essential to remove the delivery tube from the water before you stop heating, otherwise you risk cold water sucking back into a very hot tube. It will crack - sometimes violently! The results You might expect aluminium to be pretty reactive given its position in the reactivity series - but it isn't. Aluminium has a very thin but very strong layer of aluminium oxide on the surface and this slows its reactions down. Even if you use aluminium powder, you will only get a slow production of hydrogen. White aluminium oxide is formed as well.
Zinc and iron behave exactly as you would expect, giving a good flow of hydrogen. Zinc oxide is formed, which is yellow when it is hot and white when it cools.
Iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, Fe3O4. The name describes the formula, triiron tetroxide - three irons and four oxygens.
Lead is another awkward one. It is just above hydrogen in the reactivity series and so you would expect a slow reaction. In fact, there is no reaction. Technically, it is above hydrogen in the reactivity series, but with the exception of a single reaction with concentrated hydrochloric acid which you will meet on the next page in this sequence, for most purposes you can count lead as being below hydrogen in the series. Actually, most modern syllabuses don't include lead in the reactivity series at all to avoid this problem. If you are working towards an exam, check your syllabus to see it you need to know about lead. If you don't, just ignore it! If you are doing a UK-based syllabus, you can find links to the Exam Boards' websites where you can download a copy of your syllabus and other useful stuff on the about this part of Chemguide page. The metals below hydrogen in the reactivity series don't react with steam. Summary Metals from calcium upwards These react vigorously (and even more vigorously the higher up the series you go) with cold water to form the metal hydroxide and hydrogen. Metals from magnesium to iron These react with steam to give a metal oxide and hydrogen. Remember that the aluminium reaction is much slower than you would expect because of its stable oxide coating. Metals below hydrogen These don't react with water under any conditions. | |||||||||||||||||||||||||||||||||||||||||||
|
Note: If you want to explore more of the University of Nottingham chemistry videos click here. For even more videos from the same source, click on the "Our YouTube Channel" link at the top of their home page. | |||||||||||||||||||||||||||||||||||||||||||
© Jim Clark 2020 |
|||||||||||||||||||||||||||||||||||||||||||