|
Chemguide: Core Chemistry 14 - 16 The Periodic Table - the Transition Metals This page introduces the transition metals (also known as transition elements) and compares some of their typical properties with Group 1 elements. It is only a quick survey and doesn't contain much detail. | |||||||||
|
Note: For those of you who will go on to do chemistry at a higher level, what are always called transition elements at this level should properly be called d-block elements - for reasons to do with the arrangement of their electrons. "Transition element" has a very precise definition which doesn't include zinc, for example, at the extreme right-hand end of the first series. An older definition would also exclude elements like scandium at the other end of the series. This is not something you need to worry about at this stage. I am just pointing it out because I hate teaching stuff that will have to be unlearnt at a higher level. | |||||||||
|
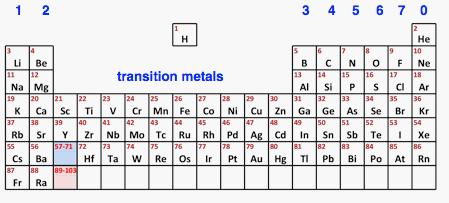
Introduction If you have been working through this section in order, you will already have come across this simplified Periodic Table.
There is a useful bit of video which covers quite a lot of what you need to know, and makes a good introduction. Physical properties Densities These vary but are much higher than the densities of the Group 1 metals. Common metals like chromium, iron, nickel and copper have densities of around 7 - 8 g cm-3 (grams per cubic centimetre). But the densities can go as high as 21.4 g cm-3 for metals like platinum. Osmium, a less familiar metal, can even beat this by a little bit. If you can't easily picture how dense some of these metals are, if you had a cube of platinum 10 cm x 10 cm x 10 cm, it would weigh 21.4 kilograms. By comparison, the Group 1 metals have densities either side of 1 g cm-3. Lithium, sodium and potassium all float on water. Melting points Again these vary, but are generally high or very high. Iron, for example, melts at just over 1500°C, and tungtsten melts at just over 3400°C. On the other hand, mercury is a liquid melting at around -39°C. So there are exceptions! The reasons for the low mercury value are well beyond this introductory level. On the other hand, Group 1 metals have relatively low melting points, ranging from 181°C for lithium to 28°C for caesium. Strength and hardness Most of the transition metals are both strong and hard. You can't cut them with a knife and, although they vary in hardness, they are all harder than Group 1 metals (except, of course, mercury again!). Remember that Group 1 metals are soft to very soft and can all be cut with a knife. Chemical properties Reactivity These metals are all much less reactive than Group 1 metals. If you think in Reactivity Series terms, the Group 1 metals are all right at the top of the series. The transition metals are found from the middle to the bottom of the series. For example, the Group 1 metals all react vigorously to violently with cold water. Some of the transition metals react with water, but very slowly. Others don't react with cold water at all - gold and platinum have no reaction with water. Iron reacts with water in the presence of air to form rust, but this is a slow process. It will react with steam but only on heating. Copper is used for water pipes, and so obviously has no simple reaction with water. It does, however, react with water very slowly in the presence of oxygen and carbon dioxide to give a green layer on the surface. You can think of the green compound as a mixture of copper(II) hydroxide and copper(II) carbonate. The photo shows this on one of the spires of Truro Cathedral which was originally covered in copper.
(Photo from Wikipedia.) Formation of several ions All Group 1 metals form a 1+ ion - and that's it! Transition metals can form a variety of ions with different charges or different formulae. The most familiar one is iron, which can form Fe2+ and Fe3+ - iron(II) and iron(III) ions. It can also form a more complicated ion where the iron is a part of a negative ion, FeO42- - the ferrate(VI) ion. (You would have to be doing chemistry at a fairly advanced level to come across that one!) Here are a few more from chromium chemistry. These are just examples of the variety possible, and you wouldn't be expected to remember them at this stage - although you might be expected to work out the charges on the first two from their names.
Formation of coloured compounds One of the most obvious characteristics of transition metals is the number of coloured compounds they form. By contrast, Group 1 metals form white or colourless compounds - unless the compound also contains a transition metal as, for example, in potassium dichromate(VI).
I originally took this photo for a GCSE book which was published in 2002. Obviously I can't remember exactly what they are, but the solutions are probably (from left to right):
Don't assume that only transition metals can form coloured compounds. You can get coloured compounds in other parts of the Periodic Table, but it is rarer. Lead(II) iodide, PbI2, for example, is a bright yellow solid, and one of the lead oxides (red lead), Pb3O4 is bright red. But only in the transition elements do you find so many elements with so many differently coloured compounds. Transition metals and their compounds as catalysts Catalysts are substances which speed reactions up but are themselves chemically unchanged at the end. There are several catalysts containing transition metals that you will meet later on in the course. They include:
© Jim Clark 2020 |
|||||||||