|
Chemguide: Core Chemistry 14 - 16 The Periodic Table - the Noble Gases This page introduces the Noble Gases in Group 0 of the Periodic Table. The modern name for Group 0 is Group 18, but I am using the older name for reasons I have already talked about on the page introducing the Periodic Table The Noble Gases
Radon is a radioactive gas produced from the radioactive decay of uranium and thorium. Physical properties of the Noble Gases Obviously their name suggests that they are all gases. They are colourless and odourless. Boiling points Their boiling points increase with the size of the atom.
The temperatures quoted are all in Kelvin, the scale starting from absolute zero. On this scale, a room temperature of 20°C is 293 K. So all of these boil at temperatures well below room temperature. Nobody will expect you to remember these figures, but you might need to explain why they get bigger as the atoms get bigger. | |||||||||||||||||||||||||
|
Important: Before you go on, it is essential that you read the page about intermolecular forces, particularly van der Waals dispersion forces. Even if you have already read this, you need it fresh in your mind for the next bit. Use the back button on your browser to come back here afterwards. | |||||||||||||||||||||||||
|
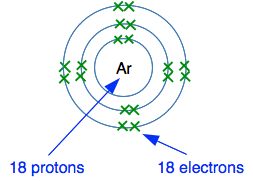
The Noble Gases are monatomic. That means that they go around as single atoms, not in pairs like oxygen and hydrogen, for example. You can picture one of their atoms as looking like this, with a positive nucleus at the centre of a number of negative electrons. Overall, the atom is electrically neutral.
But this is misleading, because it ignores the fact that the electrons are moving around within the atom, and at any one time they might be more unevenly arranged. That would give the atom an electrical dipole with the nucleus no longer at the centre of the electrons. Where the electrons are densest there will be a slight negative charge, δ-, and the region around the nucleus a slight positive charge, δ+.
If another atom came close to this one, you would get a similar dipole induced in the second one, and they would attract each other. The electrons in both atoms are, of course, still moving, and the nature of the dipoles will be constantly changing, but as long as the atoms were close together, they would change in synchronisation with each other. If the atoms were travelling slowly enough, they might stick together and start to form a liquid. The size of the temporary fluctuating dipole you can get depends on the size of the atom. The larger the atom, the more electrons there are to move around, and the more volume they have to move around in. In a very small atom like helium, the dipoles formed are tiny. The atoms won't start to stick together to form a liquid until you get a temperature close to absolute zero. As you go down the group, the atoms get bigger, and the temporary fluctuating dipoles increase. So the boiling points increase. Density The table shows the densities of the gases at 0°C and 1 atmosphere pressure. The densities are in units of grams per litre.
(Figures from LibreTexts.) The density increases as you go down the group. The next bit of video shows this. Unfortunately, you can't embed this in another site, and so you will have to visit YouTube. Why does the density increase? There is a law in chemistry called Avogadro's Law which says that at the same temperature and pressure, equal volumes of gas contain equal numbers of molecules. So if you have a litre of any of these gases, they contain the same number of molecules (or, in this case, atoms, because their "molecules" only have one atom.) As the atoms get bigger, they get heavier, and so you have an increasing mass in the same volume - the density goes up. | |||||||||||||||||||||||||
|
Note: There is a problem here with the modern definition of a molecule which says that it has to contain two or more atoms. That means that technically you can't call the Noble Gas particles molecules. An older definition would allow you to do that. I find it really annoying not to be able to use the word molecule with respect to Noble Gases! | |||||||||||||||||||||||||
|
Chemical properties of the Noble Gases At this level, students often get the idea that Noble Gases are completely unreactive because they have "full" electron energy levels. This isn't entirely true! XeF2 was discovered in 1962 and formed as a white solid simply by reacting xenon and fluorine in the presence of sunlight. Subsequently other compounds of xenon were discovered mainly involving fluorine and oxygen. Similar compounds of krypton have also been made. Out of interest only, this is how the XeF2 molecules are arranged in the solid.
This diagram comes from Wikipedia. However, helium, neon and argon don't form simple compounds like this. Why are Noble Gases so reluctant to form compounds? This next bit goes beyond what is normally taught at this level, and you can treat it as "just for interest". It isn't enough to say that Noble Gases don't form compounds because they have full outer energy levels. You have to explain why that matters, and why there are exceptions. Think about argon as fairly typical. Its electronic structure is 2,8,8.
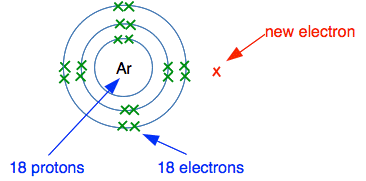
What would happen if it tried to form a negative ion by accepting another electron from somewhere - shown in the diagram in red?
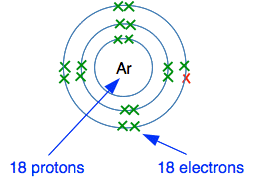
What is there to attract the new electron to the nucleus? There is no room in the third level as shown, and the new electron would have to go into a higher energy level. There are 18 protons in the nucleus, but these are surrounded by 18 electrons. The electrons cancel out the effect of the protons. There is no net charge from the nucleus to hold the new electron in place. Argon can't form negative ions. What would happen if argon tried to form a positive ion? Think about the electron in red in the next diagram. How easy would it be for that to be transferred to something else?
That electron feels the pull of 18 protons in the nucleus offset by the 10 inner electrons - that's a net pull of 8+ from the centre of the atom. It needs huge amounts of energy to remove an electron from argon, and so it can't form positive ions. What about forming covalent bonds? To form covalent bonds you need single electrons, and all argon's electrons are already paired. Well, if that's the case, how can xenon and krypton form covalent bonds? It is possible to get single electrons in these by using slightly higher electron energy levels in the atom. In xenon one of the pairs of electrons in the outer level can split, pushing one of the electrons into a higher energy level. That leaves two unpaired electrons to use to form two covalent bonds, and in fact xenon can do the same thing with one or two other electron pairs as well to give up to 6 unpaired electrons. This is how molecules like XeF2, XeF4 and XeF6 arise. Why don't helium, neon and argon do the same thing? It costs energy to move electrons into higher energy levels, and this only works if the energy gap between the levels is fairly small. In helium, neon and argon, the gap is too big. As atoms get bigger with more layers of electrons, the energy gaps between the levels gets smaller.
© Jim Clark 2020 |
|||||||||||||||||||||||||