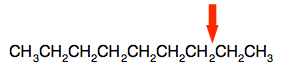|
Chemguide: Core Chemistry 14 - 16 Cracking long hydrocarbons to make smaller useful ones This page is about how you can break longer hydrocarbons into more useful shorter ones. It looks at a simple experiment to demonstrate cracking in the lab as well as some industrial details. Two important words
Why crack hydrocarbons? There are two problems with the output from fractional distillation:
What happens during cracking? Big molecules are broken at a carbon-carbon single bond. For example, consider the molecule nonane, C9H20.
We always draw these hydrocarbons as if they were tidy straight chains, but they aren't. They can rotate freely about all the carbon-carbon single bonds, and can take up all sorts of different shapes. That's why I have drawn this random one above. But we write them tidily as:
Now imagine it gets broken here:
That would produce these two fragments:
But these fragments aren't viable as they stand. If you think about the carbon atoms in the two CH2 groups at the broken ends, each of those carbon atoms is only forming 3 bonds - 1 to another carbon atom, and 2 to two hydrogen atoms. There needs to be some rearranging of the atoms. If a hydrogen atom from the far right CH3 group transfers to the CH2 group at the end of the left-hand fragment, you get this:
The two carbon atoms in the right-hand fragment can satisfy their bonding requirements by forming a double bond. So you have ended up with a smaller alkane, and a usefully reactive alkene. Should you want to write an ordinary equation for this, it would be
There's no reason, of course, why the nonane should only break at this point. It might break off a 3-carbon fragment from the right-hand end instead, so you could get:
The structural formula for C3H6 is CH2=CHCH3. It is different alkene. There are other possibilities, including it breaking more than once. Don't worry about this! A simple exam question might just give you this partial equation and ask you to complete it.
It will be a balanced equation. Count the carbons and hydrogens on the left-hand side, and add a new hydrocarbon with enough of each to balance it. In this case, you need to add another 3 carbon atoms and 6 hydrogens.
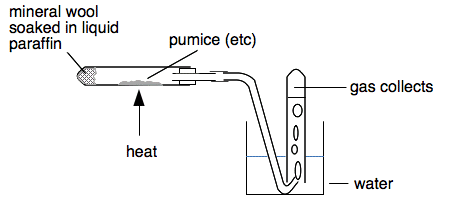
A simple demonstration of cracking in the lab "Liquid paraffin" is a colourless oily liquid which contains alkane molecules with around 20 carbon atoms. It is mainly used medically as a laxative. You can crack it easily by passing its vapour over a range of very hot catalysts including pumice stone, broken porcelain bits or aluminium oxide. You can show that an alkene (with a carbon-carbon double bond) is formed by collecting the gases produced over water and testing them. A simple experimental set-up would look like this.
You start by heating the catalyst very strongly until it is really hot. Then flick the Bunsen back to the liquid paraffin to produce some vapour. Then back to the catalyst - and keep on repeating this. You can discard the first test tube of gas collected because it will just be air expanded out of the horizontal tube on heating, or simply don't collect the first gas that comes through. When you have several tubes of gas collected, you remove the delivery tube from the water, and then stop heating.
It is essential to remove the delivery tube from the water before you stop heating, otherwise there is a risk of the water "sucking back" into the very hot test tube and cracking it. Testing the gas to show that it contains an alkene When the oil is cracked, you will have a mixture of products including shorter alkanes and probably more than one alkene. Most of these will have boiling points high enough that they will just condense to liquids in the water, and form a very thin invisible layer on top. The gases will consist mainly of small alkenes like ethene. There are two simple tests you can do:
We will look at the chemistry of this when we talk about alkenes on another page. The video shows this happening. It doesn't name the catalyst, but I suspect it is aluminium oxide. It is probably unlikely that you will need to know about the effect on acidified potassium manganate(VII) solution, but check your syllabus. Cracking industrially Most UK syllabuses just mention catalytic cracking. Check your syllabus to see if it also mentions steam cracking. Some syllabuses don't specify which version of cracking they want - in which case, look at past papers and mark schemes. There is also another method known as thermal cracking. No syllabus I have looked at mentions that. Catalytic cracking Catalytic cracking uses a zeolite as the catalyst. At this level, they are often just treated as a mixture of aluminium oxide (alumina) and silicon dioxide (silica). Catalytic cracking frequently uses the gas oil (diesel oil) fraction from crude oil fractional distillation. The gas oil is vaporised and brought into contact with the catalyst at a temperature of about 500°C and moderately low pressures. The zeolites used in catalytic cracking are chosen to give high percentages of hydrocarbons with between 5 and 10 carbon atoms, particularly useful for petrol (gasoline), as well as alkenes. Steam cracking Steam cracking is useful because it produces a high proportion of alkenes (hydrocarbons with carbon-carbon double bonds) in the cracked mixture. It uses the naphtha or (sometimes) gas oil fraction as the feedstock as well as more simple hydrocarbons like ethane, propane or butane. These are vaporised and mixed with steam and passed through a reactor heated to about 800 - 900°C. The pressure of the mixture is around 1 atmosphere. The gas flow is very, very fast so that the mixture only remains in the reactor for less than a second. This is to prevent the hydrocarbons cracking to produce carbon. The steam plays no chemical part in the process, serving mainly to dilute the organic feedstock. This helps to prevent the formation of carbon. | |
|
Note: If you are interested, there is a lot of really useful information on cracking on this page from the Essential Chemical Industry. You will be reading that out of interest, not because you need to know it all! | |
© Jim Clark 2021 |
|