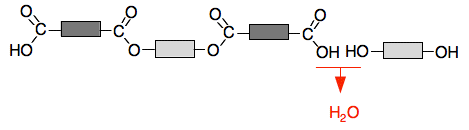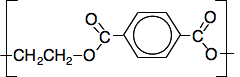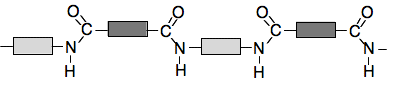|
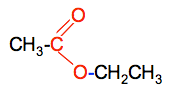
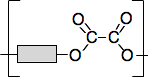
Chemguide: Core Chemistry 14 - 16 Condensation Polymerisation This page is an introduction to condensation polymerisation and the formation and structures of polyesters and nylon. I am assuming that you are familiar with esters. It would be helpful if you first read the page about addition polymerisation. That is easier to understand and introduces some key terms. Polyesters Polyesters are polymers which contain ester links holding the chain together. We need to start by looking at the formation of a simple ester again. A close look at the bonding in esters You will remember that an ester is formed when a carboxylic acid reacts with an alcohol in the presence of some concentrated sulfuric acid as a catalyst. For example, the equation for the formation of ethyl ethanoate is: CH3COOH(l) + CH3CH2OH(l) The two hydrocarbon groups are being held together with what we might call an "ester link". This link is shown in red in the next diagram.
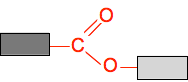
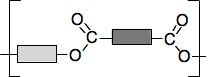
It doesn't matter what you do to change the hydrocarbon groups, the ester link will always be the same. So we can generalise this with what is known as a "block diagram".
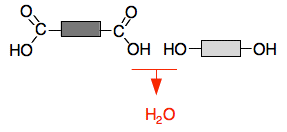
The darker grey block could be methyl or ethyl or propyl or any other hydrocarbon group, and could also just be a hydrogen atom if the ester came from methanoic acid. Similarly, the paler block could be any hydrocarbon group - but not a hydrogen this time. If it was a hydrogen atom, then it wouldn't be an ester any longer. It would be a carboxylic acid. Making a chain with ester links Obviously, if you have got an ester like the one in the diagrams above, there is no way you could make a chain out of it. But suppose you started with an acid which had two COOH groups and an alcohol with two OH groups. Using block diagrams again:
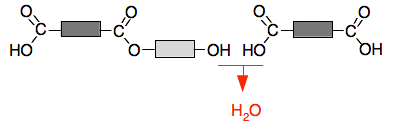
An ester link will form by losing a water molecule from a combination of the OH group and the COOH group. That gives:
Now suppose another of the acid molecules approaches the right-hand side:
You can get another ester link formed:
Now suppose another of the alcohol molecules approches the right-hand side:
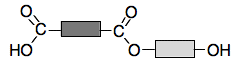
And so on, and so on . . . We are building up a polymer chain, but this time using two monomers. The repeat unit contains both monomers.
Be careful when you draw this to include all the repeat unit, but without including bits of the next one. You will find various different versions of the repeat unit depending on where the author chose to start. All that matters is that you include all the atoms or groups you need to repeat, and nothing else. Why is this called condensation polymerisation? In organic chemistry a condensation reaction is one where two molecules join together with the loss of a small molecule when that happens. In this case, every time you create a new ester link, a water molecule is lost. That's different from addition polymerisation where you join molecules up with nothing being lost. Despite the word "condensation", the small molecule lost doesn't have to be water. Looking at a real example The molecule with the two OH groups is usually ethane-1,2-diol: HOCH2CH2OH. Molecules like this are often called diols - that just means two OH groups. Benzene-1,4-dicarboxylic acid (old name: terephthalic acid) is frequently used as the molecule with two COOH groups. The structure of benzene is difficult to explain at this level. It has 6 carbon atoms in a ring with one other atom or group attached to each carbon atom. it is usually drawn as a hexagon with a ring in the middle. If the other things attached to the ring are hydrogens, we don't bother to write them in - we just write in other groups like COOH groups in this instance.
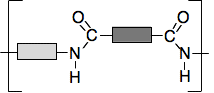
So you can just slot these into the block diagram we have built up. The pale grey block can be replaced by CH2CH2, and the dark grey one by the benzene ring. The repeat unit is
This is the very common polymer know as polyester (if it is used in clothing) or PET (if it is used to make drink bottles, for example). In clothing, it is sometimes known by the brand name Terylene. Some final thoughts about polyesters This is a case where it is essential that you look at past exam papers and mark schemes to find out what sort of questions your examiners are likely to ask you. The main UK Exam Boards are listed on the page about this 14 to 16 section. It may well be that your examiners will give you the formulae for the diacid and diol that you need. So you just substitute the bits of the formulae you need into this general block diagram for the repeat unit you have seen above.
In most cases, the diol will be HOCH2CH2OH. So the bit replacing the pale grey box will be CH2CH2. But whatever your formula is, you replace the grey box with everything apart from the two OH groups. And similarly with the diacid. If you were given the acid HOOCCH2COOH, the dark grey box would be replaced with CH2 - in other words everthing apart from the two COOH groups, which are already in the formula as the COO groups. There is one more case that I need to point out to you because it could upset you if you hadn't come across it before. The simplest acid with two COOH groups is ethanedioic acid, HOOCCOOH. So there is nothing between the two COOH groups. What do you replace the dark grey box with? Nothing! That part of the repeat unit would now look like this:
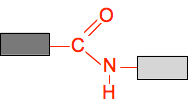
Polyamides - nylon Before you get bogged down in this, check your syllabus to be sure you need to know about it. Amide links This introduces a bit of chemistry that you won't have met before, and is only needed for this topic. We have looked already on this page at ester links, including the block diagram for a simple ester:
An amide link is similar, but replaces the bottom oxygen by a N-H group.
Making a chain with amide links - polyamides I am not going to talk this through from scratch. Instead I am going to assume that you have worked carefully through the beginning of the page about polyesters and just make the small modification needed to that. This time you start as before with a diacid - a molecule with two COOH groups. But instead of a diol, you use a compound with two NH2 groups in it. There are various forms of nylon which vary with the lengths of the carbon chains in these two molecules. Nylon-6,6 is made from:
This is nylon-6,6 because each of the monomers it is made from contains 6 carbon atoms. You are almost certainly not going to need to remember this if you are doing a UK exam. The two syllabuses which wanted polyamides at the time of writing just wanted block diagrams. The repeat unit in a polyamide chain would be:
. . . and a very short bit of chain would look like this:
This is exactly like the polyester repeat unit and chain except that the single-bonded oxygen atom in the COO group is replaced by NH. Making nylon in the lab To get a rapid reaction in the lab, we speed things up by replacing the acid with an acyl chloride (also known as acid chlorides). In these molecules, the OH group in the COOH group has been replaced by chlorine to give a COCl group. Acyl chlorides are far more reactive than carboxylic acids. In the bit of video below, it shows how you can make nylon-6,10 as a simple demonstration in the lab. The materials used are:
If you count them, there are now 10 carbon atoms in the acyl chloride. (This is out of interest only - you won't be expected to remember it.) This time, the small molecule being lost is HCl rather than water. In the video, ignore the names - you won't need them for exams at this level. I just want you to look at the reaction.
© Jim Clark 2021 |