|
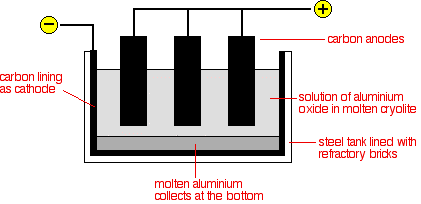
Chemguide: Core Chemistry 14 - 16 Aluminium This page looks at the extraction of aluminium from purified bauxite, together with some uses of aluminium. Introduction Aluminium is too high in the reactivity series to extract it from its ore using carbon reduction. The temperatures needed are too high to be economic. Instead, it is extracted by electrolysis. The ore is first converted into pure aluminium oxide, and this is then electrolysed in solution in molten cryolite - another aluminium compound. The aluminium oxide has too high a melting point to electrolyse on its own. At this level, you are unlikely to need to know how the aluminium oxide is obtained from the bauxite. Aluminium ore The usual aluminium ore is bauxite. Bauxite is essentially an impure aluminium oxide. The major impurities include iron oxides, silicon dioxide and titanium dioxide. Conversion of the aluminium oxide into aluminium by electrolysis The aluminium oxide is electrolysed in solution in molten cryolite, Na3AlF6. Cryolite is another aluminium ore, but is rare and expensive, and most is now made chemically. The electrolysis cell Start by watching a bit of video which gives some idea of the scale of production. The diagram shows a very simplified version of an electrolysis cell.
Although the carbon lining of the cell is labelled as the cathode, the effective cathode is mainly the molten aluminium that forms on the bottom of the cell. Molten aluminium is syphoned out of the cell from time to time, and new aluminium oxide added at the top. The cell operates at a low voltage of about 5 - 6 volts, but at huge currents of 100,000 amps or more. The heating effect of these large currents keeps the cell at a temperature of about 1000°C. The electrode reactions These are very complicated. For chemistry purposes at this level, they are always simplified. This is the simplification: Aluminium is released at the cathode. Aluminium ions are reduced by gaining 3 electrons.
Oxygen is produced initially at the anode.
| |||||||||||
|
Note: The equations on the video are scaled up so that 12 electrons are transferred over-all. In an exam, if you are asked for the electrode equations, keep it simple as above. | |||||||||||
|
However, at the temperature of the cell, the carbon anodes burn in this oxygen to give carbon dioxide and carbon monoxide. Continual replacement of the anodes is a major expense. Uses of aluminium Aluminium is usually alloyed with other elements such as silicon, copper or magnesium. Pure aluminium isn't very strong, and alloying it adds to it strength. Aluminium is especially useful because it
Some uses include:
© Jim Clark 2020 |
|||||||||||