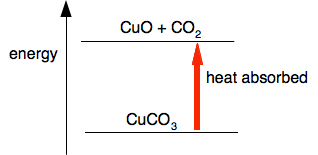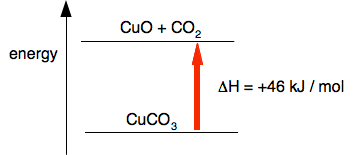|
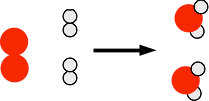
Chemguide: Core Chemistry 14 - 16 Introducing energy changes during reactions A lot of reactions produce heat when they happen. Other reactions need heat to make them happen. This page looks at why this is, and introduces some key chemistry terms. Why do chemical changes involve energy changes? All chemical reactions involve breaking some bonds and making others. These may be covalent bonds or ionic bonds or bonds involving intermolecular attractions - it doesn't matter what sorts of bonds are involved. Every chemical change involves breaking some bonds and making others. Consider the reaction between hydrogen and oxygen to make water. A huge amount of energy is produced when this happens. You may have already seen this bit of video on an earlier page showing a balloon containing a mixture of hydrogen and oxygen being ignited. In the balloon, this reaction between hydrogen and oxygen is happening.
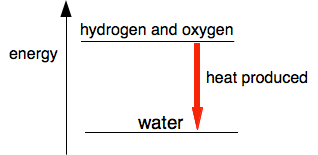
Where is the energy coming from? You need to break bonds between oxygen atoms and between hydrogen atoms. That needs an input of quite a lot of energy. But making bonds releases energy, and you get a lot more energy out when the hydrogen-oxygen bonds are made than you had to put in to produce the hydrogen and oxygen atoms. Where does the energy come from to split up the hydrogen and oxygen molecules? That energy initially comes from the flame or spark that you use to start the reaction. But as the reaction happens, formation of the new bonds releases enough energy to break more oxygen and hydrogen molecules up - with a lot of energy to spare. Energy diagrams Where heat is given off by the reaction We can draw a simple diagram for this reaction showing the change in energy.
The hydrogen and oxygen are losing energy to form water. That energy is transferred to the surroundings, and so the surroundings get hot. If you do this reaction in a controlled way by lighting a jet of hydrogen in air or oxygen, then you can use this as a source of heat.
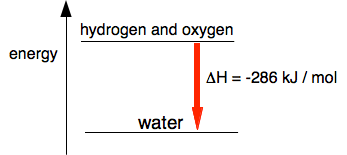
The water is energetically more stable than the mixture of hydrogen and oxygen. To get the water to split up again takes a lot of energy. We can measure the heat given off by reactions in units of kilojoules per mole, written as kJ / mol or kJ mol-1. The mole (abbreviated as "mol") is a measure of a standard amount of a substance. You will meet that in detail when we eventually look at some chemistry calculations. Don't worry about it for now. The amount of heat evolved or absorbed during a reaction is given the symbol ΔH. You say this "delta H". The symbol Δ stands for "change in". So ΔH is the change in heat. ΔH is recorded from the point of view of the substances taking part, and in the oxygen-hydrogen reaction, those substances are losing energy to the surroundings. So for an exothermic reaction, ΔH is negative. When hydrogen and oxygen combine to make water, ΔH is -286 kJ per mole of water formed. So, modifying the last diagram to include the value of the heat evolved:
Where heat is absorbed during the reaction A simple case of heat being absorbed is in thermal decomposition reactions. Thermal decomposition means splitting a compound up into smaller pieces by heating it. This video shows the effect of heat on solid copper(II) carbonate.
In the absence of heat, this reaction doesn't work. You don't just have to heat it to make it start, you have to heat it continuously. A simple energy diagram would look like this:
You have to supply heat energy in order to turn copper(II) carbonate into copper(II) oxide and carbon dioxide.
A calculated value for the amount of heat absorbed during the reaction showed that ΔH was +46 kJ per mole of copper(II) carbonate broken up. So, modifying the last diagram:
A positive sign for ΔH shows that heat is being absorbed. The reaction has had to gain energy in order to happen. Summary of what you need to know
© Jim Clark 2020 |