|
Chemguide: Core Chemistry 14 - 16 Subatomic Particles, the Nucleus and Isotopes This page looks briefly at the three subatomic particles we talk about at this level (protons, neutrons and electrons), and then goes on to look at how you work out the numbers of protons and neutrons in the nucleus. It finishes by looking at the existence of isotopes of elements. Protons, neutrons and electrons The table summarises the key facts about these particles.
What you need to notice:
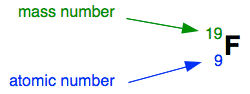
The nucleus The nucleus is at the centre of the atom and contains the protons and neutrons. Protons and neutrons are collectively known as nucleons. You aren't very likely to meet this term at this introductory level. Virtually all the mass of the atom is concentrated in the nucleus, because the electrons weigh so little. Working out the numbers of protons and neutrons No of protons = ATOMIC NUMBER of the atom The atomic number is also given the more descriptive name of proton number, and is related to the position of the element in the Periodic Table. No of protons + no of neutrons = MASS NUMBER of the atom The mass number is also called the nucleon number - again you probably won't meet that at this level. Using fluorine as an example, this information can be given simply in the form:
How many protons and neutrons has this atom got?
The atomic number is tied to the position of the element in the Periodic Table, and so the number of protons defines what sort of element you are talking about. So if an atom has 8 protons (atomic number = 8), it must be oxygen. If an atom has 12 protons (atomic number = 12), it must be magnesium. Similarly, every argon atom (atomic number = 18) has 18 protons; every uranium atom (atomic number = 92) has 92 protons. Another example - working out the number of protons and neutrons in Br-79 Br-79 is another way of writing 79Br. So bromine-79 is a bromine atom with a mass number of 79. First you need to find bromine, Br, in the Periodic Table. You really need a printed version of this, and will find a simple downloadable version from this site. The download button is at the beginning of the second paragraph under the table.
Some warnings! Periodic Tables will normally give you two numbers for each element. The atomic number is the smaller number - don't get the two numbers confused. The other number is NOT the mass number - it is the relative atomic mass of the element, which in most cases won't be the same as the mass number. In a Periodic Table such as the one I have suggested, which gives the relative atomic mass to one decimal place, it is obvious that a number like 79.9 (which is given for bromine in this table) can't be the mass number. The total number of protons plus neutrons has to be a whole number. Except for one common example which you might be expected to remember, in an exam you would normally be given the mass number of the atom if you need it - as I have done at the top of this example. We will talk some more about relative atomic mass in a moment. Isotopes The number of neutrons in an atom can vary within small limits. For example, there are three kinds of carbon atom 12C, 13C and 14C. They all have the same number of protons, but the number of neutrons varies.
These different atoms of carbon are called isotopes. The fact that they have varying numbers of neutrons makes no difference whatsoever to the chemical reactions of the carbon. Isotopes are atoms which have the same atomic number but different mass numbers. They have the same number of protons but different numbers of neutrons. Isotopes and relative atomic mass This isn't intended to be a proper introduction to relative atomic mass - just a brief look at how it relates to isotopes. An example using chlorine This is the example where the numbers are so easy that you might well be expected to remember them. Chlorine has two isotopes, Cl-35 and Cl-37, and ordinary chlorine contains these in the ratio of 3 atoms of Cl-35 to every 1 atom of Cl-37 (to a good-enough approximation for our purposes). If you have a sample of chlorine it will contain unbelievably vast numbers of chlorine atoms, and it is useful to be able to give an average value for the mass of a chlorine atom. An average of 35 and 37 is 36, but that doesn't allow for the fact that there are three times as many Cl-35 atoms as Cl-37. Relative atomic mass is a weighted average (often called a weighted mean) of the masses of the isotopes. That is an average which takes account of the different proportions of the various isotopes. Suppose you had four typical chlorine atoms - 3 atoms of Cl-35 and 1 atom of Cl-37. The total mass of the four atoms would be (3 x 35) + (1 x 37) = 142 The "average" mass of one atom is therefore 142/4 = 35.5 If you look at the Periodic Table you will find that 35.5 is the figure quoted as the relative atomic mass of chlorine. In case you are wondering about the units of relative atomic mass - there aren't any! It is measured on a scale on which the carbon-12 isotope has a mass of exactly 12 units. You don't need to worry about that at the moment. | |||||||||||||||||||||||||||||
|
Note: You will find a proper discussion of relative atomic mass in the Calculations section of this site on this page. | |||||||||||||||||||||||||||||
© Jim Clark 2019 (modified May 2021) |
|||||||||||||||||||||||||||||