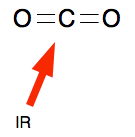|
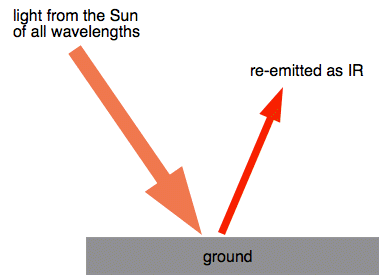
Chemguide: Core Chemistry 14 - 16 Environmental problems involving the air - the greenhouse effect This page looks at the greenhouse effect and the role of carbon dioxide in global warming. Start by watching three linked videos which give a pretty good survey of the topic. The greenhouse effect (summary) When light from the Sun strikes the ground, it warms it. Some of that energy from the Sun is then re-emitted as infra-red (IR) radiation.
Infra-red light can interact with a lot of molecules, making the bonds vibrate faster. You mustn't imagine that bonds between atoms in a molecule are rigid - think of them more like springs which can compress and expand or bend. Carbon dioxide interacts with IR light in this way. The energy of the light is absorbed by the bonds in the molecule.
That energy can be transferred to other molecules in the air during collision. The result of lots of interactions between carbon dioxide and IR light, and then collisions of this sort, is to increase the energy of all sorts of different molecules in the atmosphere. An increase in energy of the molecules in this way is observed as an increase in the temperature of the atmosphere. You mustn't think that carbon dioxide is the only molecule that can absorb IR light in this way. Several other molecules in the atmosphere can also do this. But the one which makes the greatest contribution is actually water vapour. Some of the IR energy absorbed by the molecules is emitted again and escapes back into space. If that wasn't the case, the atmosphere would just go on heating up constantly. The balance between absorption of energy and re-emission of energy is what keeps the Earth at a temperature suitable for life. Increasing carbon dioxide levels In the 50 years from 1970 to 2020, the percentage of carbon dioxide in the atmosphere increased from 0.0325% to 0.0414%. That's an increase of a bit more than a quarter in just 50 years. The more carbon dioxide there is, the more infra-red light can get absorbed and the hotter the atmosphere can get. Sources of the extra carbon dioxide The major sources are:
Effects
© Jim Clark 2020 |