|
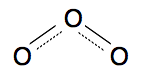
Chemguide: Core Chemistry 14 - 16 Environmental problems involving the air - ozone depletion in the high atmosphere This page looks at the so-called hole in the ozone layer - what causes it and the effect it has. If you are preparing for an exam, this probably won't be on your syllabus, but it is still worth reading about out of interest. Ozone in the high atmosphere (the stratosphere) There are two videos which give a good survey of the topic suitable for this level. There is, however, a flaw in these videos in the structure of ozone that they use. Ozone is a form of oxygen with the formula O3, unlike ordinary oxygen which is O2. The structure in the videos shows ozone as an oxygen molecule (O=O) sometimes with an extra oxygen loosely tacked on to it, and sometimes with it bonded to the oxygen molecule more strongly, but with two different sorts of bonds involving the oxygens. Both versions are misleading. A better picture of what it actually looks like is
It is impossible to explain the bonding in ozone properly at this level, but you can see that it is V-shaped molecule with two identical bonds joining the three oxygen atoms. It is an unstable molecule, and falls to pieces easily to give an ordinary oxygen molecule and an oxygen atom. That's all you need to know. The first video talks about the atmosphere and how ozone is formed in the high atmosphere (the stratosphere). The second video looks at how ozone can get destroyed. We will talk about this some more when you have watched the video. Natural formation and breaking up of ozone in the stratosphere. Formation of ozone When ultra-violet light (UV) hits an oxygen molecule, the energy in the light can break the molecule up into its two atoms.
The dots next to the oxygen atoms show unpaired electrons. Any atom or group of atoms which has one or more unpaired electrons is called a free radical. The oxygen radical can lose energy by collision with other molecules in the atmosphere. When it is slow enough it can attach to an oxygen molecule that it hits to make an ozone molecule.
Break-up of ozone This again involves UV light. Ozone isn't a very stable molecule, and can be broken up again by the energy in UV light.
Notice that both the formation and break-up of ozone involve UV light. The energy in that light is used to carry out chemical changes. These are called photochemical reactions - a term use in the videos. Over-all These two reactions are going on all the time, and are in balance with each other so that the amount of ozone in the high atmosphere remains constant. Since the UV energy is used up doing these reactions, it can't reach the surface of the Earth. That is a good thing because UV can cause skin cancer and inhibit plant growth. The ozone layer protects against this. The effect of pollution Chlorofluorocarbons (CFCs) such as CCl2F2 were widely used as the coolants in fridges and freezers, for expanding polystyrene and as the propellant in aerosol cans. They have now been replaced by less harmful things. In the atmosphere, CFCs can split up and form chlorine radicals - simple chlorine atoms Cl· - and these can react with ozone.
The ClO· formed is also a free radical. If this hits another ozone molecule, this can happen
Two important things have happened:
Reactions like this are known as chain reactions. Ozone as a pollutant in the lower atmosphere Ozone is poisonous, and is formed by the effect of sunlight on other pollutants present in vehicle emissions, such as NO2 and VOC. VOC stands for Volatile Organic Compounds - at its most simple, unburnt molecules from the petrol (gasoline) or diesel oil. The chemistry of this is too complicated to deal with at this level. | |
|
Note: The chemistry above is more commonly talked about in advanced chemistry courses, and it is unlikely that you would be asked about it in an exam at this introductory level. But it is interesting, and not very difficult, and there are some things that are just good to know about! | |
© Jim Clark 2020 |
|