|
Chemguide: Core Chemistry 14 - 16 Making oxygen in the lab This page shows a simple way of making oxygen in the lab from hydrogen peroxide. In truth, if you want oxygen in the lab, you almost invariably just take it from an oxygen cylinder. This shows how you might do it if you just wanted a small amount. It also looks at the test for oxygen. The catalytic decomposition of hydrogen peroxide Hydrogen peroxide, H2O2, is like a water molecule with an extra oxygen atom. It is fairly unstable and its solution breaks up to give water and oxygen gas.
This happens very slowly all the time, but can be speeded up considerably by adding a catalyst, manganese(IV) oxide, MnO2. A catalyst speeds up a reaction but can be recovered chemically unchanged at the end of it.
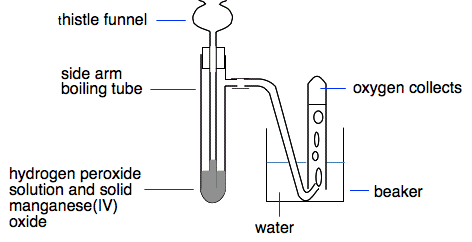
A small amount of manganese(IV) oxide is put in the bottom of the side arm boiling tube, and at least enough hydrogen peroxide solution is poured down the thistle funnel to cover the bottom of the funnel. There is rapid fizzing as oxygen is produced, and this can be collected over water. Testing for oxygen Things burn much better in pure oxygen than in air, and the test makes use of this. If you put a glowing wooden splint into oxygen, the splint will relight. This is the test for oxygen. You can see that the splint bursts into a very bright flame again. It is possible that you might have noticed a slight pop as it relit. That is quite common, and it is important not to think that there is hydrogen there! The test for hydrogen is to put a lighted spint in and get a very squeaky pop. The pop with oxygen is more noticeable if you are using a bigger volume of oxygen. The next bit of video shows this happening, and explains why the pop happens. Most textbooks and teachers won't mention this. I am including it in case you get confused with the pop from hydrogen. What is absolutely important is that oxygen relights the splint. Hydrogen won't do that. For exam purposes, the test for oxygen is simply that it relights a glowing splint.
© Jim Clark 2020 |