|
Chemguide: Core Chemistry 14 - 16 The composition of the air This page looks at the proportions of the various gases in the air - in particular, nitrogen, oxygen, argon and carbon dioxide. It also gives two simple ways of finding the percentage of oxygen in the air. The main gases in the air The table shows the proportions of the main gases in dry unpolluted air, rounded to two decimal places.
There are also other gases present in even smaller amounts - like neon and krypton, for example. But there is another gas whose percentage varies according to the conditions. That is water vapour. In hot humid places on Earth that can be as much as 4%. We tend to simplify these figures for general use:
Finding the percentage of oxygen in the air The classic method of doing this is to pass 100 cm3 of air backwards and forwards over heated copper packed tightly into a glass or silica tube. A silica tube looks like glass, but is much more resistant to melting. The air is pushed backwards and forwards across the heated copper until there is no further reduction in volume. The apparatus is allowed to cool because hot air expands, and the volume remaining is measured. Some of the copper in the tube turns black as it turns to copper(II) oxide.
It is important that there is an excess of unchanged copper in the tube at the end so that you can be sure that the reaction stopped because the oxygen was all used up. The volume left is 79 cm3, showing that air contains 21% oxygen. | |||||||||
|
Just out of interest: This experiment is almost always described without any criticism, and people are always pleased that it seems to produce such a good result. However, there are two potential errors in it. The first is that, however tightly you pack the copper into the tube, there will always be a certain amount of unmeasured air between the bits of copper, in the rubber tubing and in the tubes at the end of the syringes. If this totalled, say, 5 cm3, the volume would have decreased by an extra 1 cm3 because of its oxygen content. The other problem is that the accepted value of about 21% for oxygen depends on it being dry air. In most parts of the world, it isn't going to be dry air - there could be up to 4% of water vapour in the air. The percentage of oxygen in damp air will be below 21% because of the space taken up by water vapour, and so the volume will decrease less than you might expect. As it happens, these errors work in opposite directions, and more or less cancel each other out. Why am I telling you all this? Because if you want to do science in the future, you have to be critical and not just accept everything you are told! If you are doing an exam, do not comment on this! | |||||||||
|
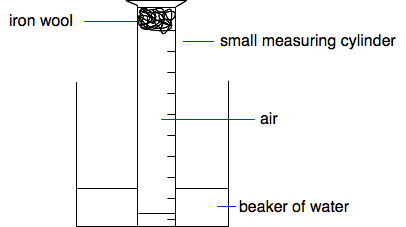
An alternative method using the rusting of iron When iron rusts, it combines with oxygen and water to produce hydrated iron(III) oxide - that's iron(III) oxide with water attached to it, Fe2O3.xH2O. "x" shows a variable amount of water. Obviously, if iron rusts, it must be removing oxygen from the air. This simple experiment uses this to find the percentage of oxygen in the air.
The iron wool is placed in a measuring cylinder and wetted by adding some water. Most of the water is poured off, but it is best to leave a little bit in the cylinder so that when you invert it over a beaker of water, the water level in the cylinder falls within the cylinder markings. You make a note of the water level in the cylinder (showing the volume of gas contained in it), and then leave it for a few days for the iron to rust.
The water level obviously rises as the oxygen is used up. Make a note of the new level. Made up results!
| |||||||||
|
Note: If you do this, you will almost certainly use steel wool rather than iron wool. This is iron which has a very small amount of carbon in it. It behaves chemically as if it were pure iron. If you were being fussy, you might argue that we haven't allowed for the space taken up in the measuring cylinder by the iron. In fact, this is going to be pretty small. You don't need much iron for the small amount of oxygen in the tube to react with. And, because it is used as a loose wool, most of its volume is air anyway. | |||||||||
© Jim Clark 2020 |
|||||||||