|
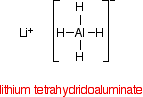
REDUCING NITRILES TO PRIMARY AMINES This page looks at the reduction of nitriles to primary amines using either lithium tetrahydridoaluminate(III) (lithium aluminium hydride) or hydrogen and a metal catalyst. The reduction of nitriles using LiAlH4 The reducing agent Despite its name, the structure of the reducing agent is very simple. There are four hydrogens ("tetrahydido") around the aluminium in a negative ion (shown by the "ate" ending). The "(III)" shows the oxidation state of the aluminium, and is often left out because aluminium only ever shows the +3 oxidation state in its compounds. To make the name shorter, that's what I shall do for the rest of this page. | ||
|
Note: It isn't important as far as the current page is concerned, but if you want to understand more about oxidation states (oxidation numbers), you will find them explained if you follow this link. Use the BACK button on your browser to return to this page. | ||
|
The structure of LiAlH4 is:
In the negative ion, one of the bonds is a co-ordinate covalent (dative covalent) bond using the lone pair on a hydride ion (H-) to form a bond with an empty orbital on the aluminium. | ||
|
Note: Follow this link if you aren't happy about co-ordinate covalent (dative covalent) bonding. Again, it isn't particularly important as far as the current page is concerned. Use the BACK button on your browser to return to this page. | ||
|
The overall reaction The nitrile reacts with the lithium tetrahydridoaluminate in solution in ethoxyethane (diethyl ether, or just "ether") followed by treatment of the product of that reaction with a dilute acid. Overall, the carbon-nitrogen triple bond is reduced to give a primary amine. Primary amines contain the -NH2 group. For example, with ethanenitrile you get ethylamine:
Notice that this is a simplified equation - perfectly acceptable to UK A level examiners. [H] means "hydrogen from a reducing agent". | ||
|
Note: If you know about the reduction of aldehydes and ketones, you may know that they are also reduced by the similar compound NaBH4. However, NaBH4 isn't a strong enough reducing agent to reduce nitriles. | ||
|
The reduction of nitriles using hydrogen and a metal catalyst The carbon-nitrogen triple bond in a nitrile can also be reduced by reaction with hydrogen gas in the presence of a variety of metal catalysts. Commonly quoted catalysts are palladium, platinum or nickel. The reaction will take place at a raised temperature and pressure. It is impossible to give exact details because it will vary from catalyst to catalyst. For example, ethanenitrile can be reduced to ethylamine by reaction with hydrogen in the presence of a palladium catalyst.
| ||
|
Note: Notice that this time the hydrogen is written normally as H2. This is a proper equation involving hydrogen gas - not a simplification. | ||
© Jim Clark 2004 (modified February 2016) |
||