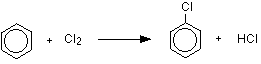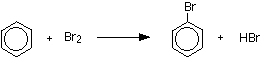|
MAKING ARYL HALIDES (HALOGENOARENES) This page only looks at the ways of making the aryl halides, chlorobenzene, bromobenzene and iodobenzene, as required by some UK A level syllabuses. It is not intended to be an overall survey of the topic. Making chlorobenzene Benzene reacts with chlorine in the presence of a catalyst, replacing one of the hydrogen atoms on the ring by a chlorine atom. The reaction happens at room temperature. The catalyst is either aluminium chloride or iron. Strictly speaking iron isn't a catalyst, because it gets permanently changed during the reaction. It reacts with some of the chlorine to form iron(III) chloride, FeCl3.
This compound acts as the catalyst and behaves exactly like aluminium chloride, AlCl3, in this reaction. The reaction between benzene and chlorine in the presence of either aluminium chloride or iron gives chlorobenzene.
or, written more compactly:
| ||
|
Note: Follow this link if you want the mechanism for the chlorination of benzene. Use the BACK button on your browser to return to this page later. | ||
|
Making bromobenzene The reaction between benzene and bromine in the presence of either aluminium bromide (rather than aluminium chloride) or iron gives bromobenzene. Iron is usually used because it is cheaper and more readily available. If you use iron, it is first converted into iron(III) bromide by the reaction between the iron and bromine.
or:
| ||
|
Note: The mechanism for this reaction is exactly the same as the chlorine reaction. Use the link further up the page if you are interested. | ||
|
Making iodobenzene Iodobenzene can be made from the reaction of benzene with iodine if they are heated under reflux in the presence of concentrated nitric acid, but it is normally made from benzenediazonium chloride solution. That's what we will concentrate on here. If you add cold potassium iodide solution to ice-cold benzenediazonium chloride solution, nitrogen gas is given off, and you get oily droplets of iodobenzene formed. There is a simple reaction between the diazonium ions present in the benzenediazonium chloride solution and the iodide ions from the potassium iodide solution.
| ||
|
Note: If you want to find out more about diazonium compounds (including their preparation and their reactions), follow this link to explore the phenylamine menu. | ||
© Jim Clark 2004 (modified March 2016) |
||