|
DEHYDRATION OF MORE COMPLICATED ALCOHOLS
This page builds on your understanding of the acid catalysed dehydration of alcohols. You have to be wary with more complicated alcohols in case there is the possibility of more than one alkene being formed. Butan-2-ol is a good example of this, with no less than three different alkenes being formed when it is dehydrated. Butan-2-ol is just an example to illustrate the problems. It is important that you understand it so that you can work out what will happen in similar cases. It would be quite impossible for you to learn what happens with every single alcohol you might be presented with. The basic facts and mechanisms for these reactions are exactly the same as with propan-2-ol. This page only deals with the extra problems created by the possibility of more than one dehydration product. | |
|
Important! What follow assumes that you are familiar with the mechanism for the dehydration of propan-2-ol. If you aren't, it is essential that you follow this link before you go on. | |
|
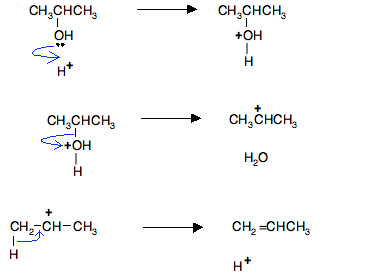
Background To make the diagrams less cluttered, we'll use the simplified version of the mechanism showing gain and loss of H+. Remember that the mechanism takes place in three stages:
So, in the case of the dehydration of propan-2-ol:
The dehydration of butan-2-ol The first two stages There is nothing new at all in these stages. In the first stage, the alcohol is protonated by picking up a hydrogen ion from the sulphuric acid. In the second stage, the positive ion then sheds a water molecule and produces a carbocation. The complication arises in the next step. When the carbocation loses a hydrogen ion, where is it going to come from? Where does the hydrogen get removed from? So that a double bond can form, it will have to come from one of the carbons next door to the one with the positive charge. If a hydrogen ion is lost from the CH3 group If a hydrogen ion is lost from the CH2 group This time the product is but-2-ene, CH3CH=CHCH3. In fact the situation is even more complicated than it looks, because but-2-ene exhibits geometric isomerism. You get a mixture of two isomers formed - cis-but-2-ene and trans-but-2-ene. Cis-but-2-ene is also known as (Z)-but-2-ene; trans-but-2-ene is also known as (E)-but-2-ene. For an explanation of the two ways of naming these two compounds, follow the link in the box below. | |
|
Geometric isomerism: Isomerism is where you can draw more than one arrangement of the atoms for a given molecular formula. Geometric isomerism is a special case of this involving molecules which have restricted rotation around one of the bonds - in this case, a carbon-carbon double bond. The C=C bond could only rotate if enough energy is put in to break the pi bond. Effectively, except at high temperatures, the C=C bond is "locked". In the case of but-2-ene, the two CH3 groups will either both be locked on one side of the C=C (to give the cis or (Z) isomer), or on opposite sides (to give the trans or (E) one). For a full discussion of geometric isomerism follow this link. Use the BACK button on your browser to return to this page. Beware! It is easy to miss geometric isomers in an exam. Always draw alkenes with the correct 120° bond angles around the C=C bond as shown in the diagrams for the cis and trans isomers above. If you take a short cut and write but-2-ene as CH3CH=CHCH3, you will almost certainly miss the fact that cis and trans forms are possible. This is a rich source of questions in an exam. You could easily throw away marks if you miss these possibilities. | |
|
The overall result Dehydration of butan-2-ol leads to a mixture containing:
To menu of elimination reactions. . . © Jim Clark 2000 (modified January 2013) |
|