|
COPPER This page looks at the extraction of copper from its ores, its purification by electrolysis, and some of its uses. Before you get too bogged down in the extraction of copper, make sure that you need it for whatever syllabus you are using. Extracting copper from its ores The method used to extract copper from its ores depends on the nature of the ore. Sulphide ores such as chalcopyrite are converted to copper by a different method from silicate, carbonate or sulphate ores. Getting copper from chalcopyrite, CuFeS2 Chalcopyrite (also known as copper pyrites) and similar sulphide ores are the commonest ores of copper. The ores typically contain low percentages of copper and have to be concentrated by, for example, froth flotation before refining. | ||
|
Note: You will find a brief description of froth flotation on the introduction to metal extraction page. | ||
|
The process The concentrated ore is heated strongly with silicon dioxide (silica), calcium carbonate and air or oxygen in a furnace or series of furnaces.
An overall equation for this series of steps is:
The copper(I) sulphide produced is converted to copper with a final blast of air.
| ||
|
Warning! This is a simplified version of the process - an attempt to condense the whole thing down to two fairly straightforward equations. The problem is that there are all sorts of variations on this extraction. One of these does the whole thing in a single furnace, and the equations above probably best represent that particular process. This quick summary is probably unsuitable for anything other than UK A level purposes. If you are working at a higher level, or want proper technical details of particular methods, you will need to look elsewhere. Try this page from The Essential Chemical Industry as a starter. | ||
|
The end product of this is called blister copper - a porous brittle form of copper, about 98 - 99.5% pure. Exploring the redox processes in this reaction It is worthwhile spending some time sorting out what the reducing agent is in these reactions, because at first sight there doesn't appear to be one! Or, if you look superficially, it seems as if it might be oxygen! But that's silly! | ||
|
Note: You aren't going to make much sense of this next bit if you don't have a good working knowledge of oxidation states (oxidation numbers). If you aren't sure, then either follow this link (which could take you some time) or skip this bit completely if you don't need to be able to do it. If you choose to follow this link, use the BACK button on your browser to return to this page later. | ||
|
I can only look at the second reaction because it is the only one I can be confident about.
Let's look at the oxidation states of everything.
That means that both the copper and the oxygen have been reduced (decrease in oxidation state). The sulphur has been oxidised (increase in oxidation state). The reducing agent is therefore the sulphide ion in the copper(I) sulphide. The other reaction is more difficult to deal with, because you can't work out all of the oxidation states by following the simple rules - there are too many variables in some of the substances. There is also apparent disagreement in the literature about whether chalcopyrite contains Cu(II) and Fe(II) or Cu(I) and Fe(III). I don't feel qualified to go any further with this. | ||
|
Note: Until I added the questions in July 2015, I had always assumed (on no good evidence - never a good idea!) that chalcopyrite contained copper and iron both in the +2 oxidation state - but that may well be wrong. If it was true, then you can show that the sulphide ions are reducing Cu(II) to Cu(I). However, if the copper was in the +1 state originally, then the sulphide ions are reducing Fe(III) to Fe(II). Because of the uncertainty, I have just deleted the paragraphs about this. It shouldn't be asked at this level anyway. | ||
|
Extracting copper from other ores Copper can be extracted from non-sulphide ores by a different process involving three separate stages:
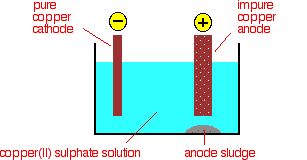
Purification of copper When copper is made from sulphide ores by the first method above, it is impure. The blister copper is first treated to remove any remaining sulphur (trapped as bubbles of sulphur dioxide in the copper - hence "blister copper") and then cast into anodes for refining using electrolysis. Electrolytic refining The purification uses an electrolyte of copper(II) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. The diagram shows a very simplified view of a cell.
At the cathode, copper(II) ions are deposited as copper.
At the anode, copper goes into solution as copper(II) ions.
For every copper ion that is deposited at the cathode, in principle another one goes into solution at the anode. The concentration of the solution should stay the same. All that happens is that there is a transfer of copper from the anode to the cathode. The cathode gets bigger as more and more pure copper is deposited; the anode gradually disappears. In practice, it isn't quite as simple as that because of the impurities involved. What happens to the impurities? Any metal in the impure anode which is below copper in the electrochemical series (reactivity series) doesn't go into solution as ions. It stays as a metal and falls to the bottom of the cell as an "anode sludge" together with any unreactive material left over from the ore. The anode sludge will contain valuable metals such as silver and gold. Metals above copper in the electrochemical series (like zinc) will form ions at the anode and go into solution. However, they won't get discharged at the cathode provided their concentration doesn't get too high. The concentration of ions like zinc will increase with time, and the concentration of the copper(II) ions in the solution will fall. For every zinc ion going into solution there will obviously be one fewer copper ion formed. (See the next note if you aren't sure about this.) The copper(II) sulphate solution has to be continuously purified to make up for this. | ||
|
Note: If it isn't obvious to you that for every zinc ion going into solution there will be one fewer copper ion, think of it like this. For each copper ion that is deposited as metallic copper at the cathode, two electrons need to flow around the circuit. Where are they coming from? Esentially, it is the anode's job to supply them. They are released there when copper or zinc atoms lose electrons and go into solution as ions. The power source then pumps them around the external circuit to the cathode. So, to deposit one copper ion at the cathode needs two electrons. These can be supplied either by a zinc atom ionising at the anode or by a copper atom ionising - it doesn't need both to happen. That means that for every extra zinc ion that gets into solution there will be one fewer copper ion going in. | ||
|
Uses of copper Amongst other things copper is used for:
To the Metal Extraction menu . . . © Jim Clark 2005 (modified July 2015) |
||