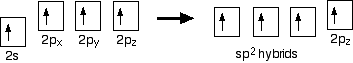|
BONDING IN ETHENE | ||
|
Important! You will find this much easier to understand if you first read the article about the bonding in methane. You may also find it useful to read the article on orbitals if you aren't sure about simple orbital theory. | ||
|
Ethene, C2H4 The simple view of the bonding in ethene At a simple level, you will have drawn ethene showing two bonds between the carbon atoms. Each line in this diagram represents one pair of shared electrons. Ethene is actually much more interesting than this. An orbital view of the bonding in ethene Ethene is built from hydrogen atoms (1s1) and carbon atoms (1s22s22px12py1). The carbon atom doesn't have enough unpaired electrons to form the required number of bonds, so it needs to promote one of the 2s2 pair into the empty 2pz orbital. This is exactly the same as happens whenever carbon forms bonds - whatever else it ends up joined to. So the first thing that happens is . . . Promotion of an electron There is only a small energy gap between the 2s and 2p orbitals, and an electron is promoted from the 2s to the empty 2p to give 4 unpaired electrons. The extra energy released when these electrons are used for bonding more than compensates for the initial input. The carbon atom is now said to be in an excited state. | ||
|
Note: If you haven't read about bonding in methane, follow this link before you go any further. Use the BACK button on your browser to come back here when you have finished. It is important that you have first met the idea of hybridisation in the more simple methane case. | ||
|
Hybridisation In the case of ethene, there is a difference from, say, methane or ethane, because each carbon is only joining to three other atoms rather than four. When the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise three of the orbitals rather than all four. They use the 2s electron and two of the 2p electrons, but leave the other 2p electron unchanged.
The new orbitals formed are called sp2 hybrids, because they are made by an s orbital and two p orbitals reorganising themselves. sp2 orbitals look rather like sp3 orbitals that you have already come across in the bonding in methane, except that they are shorter and fatter. The three sp2 hybrid orbitals arrange themselves as far apart as possible - which is at 120° to each other in a plane. The remaining p orbital is at right angles to them. The two carbon atoms and four hydrogen atoms would look like this before they joined together: The various atomic orbitals which are pointing towards each other now merge to give molecular orbitals, each containing a bonding pair of electrons. These are sigma bonds - just like those formed by end-to-end overlap of atomic orbitals in, say, ethane. The p orbitals on each carbon aren't pointing towards each other, and so we'll leave those for a moment. In the diagram, the black dots represent the nuclei of the atoms. Notice that the p orbitals are so close that they are overlapping sideways. This sideways overlap also creates a molecular orbital, but of a different kind. In this one the electrons aren't held on the line between the two nuclei, but above and below the plane of the molecule. A bond formed in this way is called a pi bond. For clarity, the sigma bonds are shown using lines - each line representing one pair of shared electrons. The various sorts of line show the directions the bonds point in. An ordinary line represents a bond in the plane of the screen (or the paper if you've printed it), a broken line is a bond going back away from you, and a wedge shows a bond coming out towards you. | ||
|
Note: The really interesting bond in ethene is the pi bond. In almost all cases where you will draw the structure of ethene, the sigma bonds will be shown as lines. | ||
|
Be clear about what a pi bond is. It is a region of space in which you can find the two electrons which make up the bond. Those two electrons can live anywhere within that space. It would be quite misleading to think of one living in the top and the other in the bottom. | ||
|
Taking chemistry further: This is another example of the curious behaviour of electrons. How do the electrons get from one half of the pi bond to the other if they are never found in between? It's an unanswerable question if you think of electrons as particles. | ||
|
Even if your syllabus doesn't expect you to know how a pi bond is formed, it will expect you to know that it exists. The pi bond dominates the chemistry of ethene. It is very vulnerable to attack - a very negative region of space above and below the plane of the molecule. It is also somewhat distant from the control of the nuclei and so is a weaker bond than the sigma bond joining the two carbons. | ||
|
Important! Check your syllabus! Find out whether you actually need to know how a pi bond is formed. Don't forget to look in the bonding section of your syllabus as well as under ethene. If you don't need to know it, there's no point in learning it! You will, however, need to know that a pi bond exists - that the two bonds between the carbon atoms in ethene aren't both the same. If you are working to a UK-based syllabus for 16 - 18 year olds, and haven't got a copy of your syllabus, find out how to download one | ||
|
All double bonds (whatever atoms they might be joining) will consist of a sigma bond and a pi bond. The shape of ethene The shape of ethene is controlled by the arrangement of the sp2 orbitals. Notice two things about them:
© Jim Clark 2000 (last modified March 2013) |
||